ABSTRACT
Hen egg is a unique and important carrier of lipid soluble bioactive carotenoids – lutein and zeaxanthin. Egg yolk carotenoid profile is largely dependent on hen’s feed composition. Naturally, lutein and zeaxanthin are polar carotenoids primarily deposited in human retina and provide several protective functions, i.e. protect the macula from damage by blue light, improve visual acuity, and scavenge harmful reactive oxygen species. They have also been linked with reduced risk of age-related macular degeneration and cataracts, cardiovascular diseases, Alzheimer’s, and possibly different cancers. This review summarizes the latest data on content and composition of hen egg carotenoids, effect of processing, feeding systems, feed additives, bioavailability, and physiological effect of egg carotenoids on human health issues.
RESUMEN
La yema de huevo es un importante y único portador de carotenoides bioactivos liposolubles: luteína y zeaxantina. El perfil de carotenoides de la yema de huevo depende notablemente de la composición alimentaria de la gallina. La luteína y la zeaxantina son carotenoides polares naturales depositados principalmente en la retina humana y que aportan diversas funciones protectoras, como por ejemplo la protección de la mácula del daño provocado por la luz azulada, la mejora de la agudeza visual y expulsan las especies dañinas reactivas al oxígeno. Estos también están relacionados con reducir el riesgo de degeneración macular producido por la edad y las cataratas, enfermedades cardiovasculares, Alzheimer, además de la posibilidad de diferentes tipos de cáncer. Este estudio resume los datos más recientes acerca del contenido y la composición de los carotenoides de la yema de huevo de gallina, el efecto del procesamiento, los sistemas de alimentación, los aditivos alimentarios, la biodisponibilidad y el efecto psicológico de los carotenoides del huevo en los problemas de salud de las personas.
1. Introduction
Lutein and zeaxanthin are the members of the carotenoid family. Carotenoids represent group of lipid soluble bioactive compounds present in wide variety of food sources. Lutein and zeaxanthin carotenoids cannot be synthesized in vivo and therefore must be obtained from the diet. Egg yolks have been reported as an important dietary source of lutein and zeaxanthin, and a range of studies have been conducted to analyze these nutrients in egg yolks, including commercial egg yolks (Goodrow et al., Citation2006; Olson, Ward, & Koutsos, Citation2008; Schlatterer & Breithaupt, Citation2006; Thurnham, Citation2007). However, under processing conditions, the highly reactive, electron-rich carotenoid molecule present in egg yolks suffers oxidation. The magnitude of oxidation depends on the amount of carotenoid present; available oxygen; exposure to light; temperature; and the presence of enzymes, metals, prooxidants, and antioxidants (Boon, McClements, Weiss, & Decker, Citation2010).
Researchers reviewed dietary sources of lutein and zeaxanthin carotenoids and their affirmative role in maintaining ocular health (Abdel-Aal, Akhtar, Zaheer, & Ali, Citation2013). Lutein and zeaxanthin may help in the prevention of macular degeneration (age-related macular degeneration [AMD]) and as such significant in reducing the risk of human eye diseases (American Optometric Association [AOA], Citation2012; Ma et al. Citation2012a; Nimalaratne, Savard, Gauthier, Schieber, & Wu, Citation2015). In addition to playing pivotal roles in ocular health, lutein and zeaxanthin are important for the prevention or reducing intensity of cardiovascular disease (CVD), stroke, cancer (Mares-Perlman, Millen, Ficek, & Hankinson, Citation2002; Shin, Xun, Nakamura, & He, Citation2013), and neurodegenerative disorders (Nataraj, Manivasagam, Justin Thenmozhi, & Essa, Citation2015). They may also be protective in skin conditions attributed to excessive ultraviolet (UV) light exposure (Blesso, Andersen, Bolling, & Fernandez, Citation2013). It is now established that there is no association between egg consumption and risk of CVD (Shin et al., Citation2013), with the exception of familial hypercholesterolemia subjects (Ruxton, Citation2010). The body needs to achieve a balance when it comes to cholesterol consumption. In this perspective, consumption of one egg per day does not increase serum cholesterol level and as such no risk of CVD among healthy men and women. Rather, egg nutrients act as the ‘enhancer’ of antioxidant defense against range of diseases.
Keeping in view the importance of lutein and zeaxanthin to human health, the laying hens may be given higher level of dietary supplementation in their feed for the purpose of increasing yolk lutein content in eggs (Leeson & Caston, Citation2004). This review summarizes the latest data on content and composition of carotenoids; effect of processing; feeding system; feed additives; bioavailability; and physiological effect of egg carotenoids on human health, especially with the availability of lutein and zeaxanthin-enriched eggs.
2. Chemistry and structural features
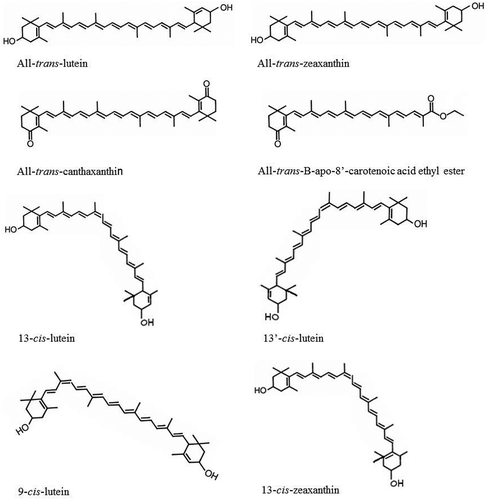
Xanthophyll carotenoids are characterizing by the presence of oxygen atoms in the molecular structure (unlike carotenes carotenoids – without oxygen atoms). Carotenoids are isoprenoids with a long polyene chain containing 3–15 conjugated double bonds, which determines their absorption spectrum. The core system of conjugated carbon–carbon bonds makes carotenoids efficient quenchers of singlet oxygen with scavenging abilities (Agarwal, Parameswari, Vasanthi, & Das, Citation2012; Fiedor & Burda, Citation2014). Also, this structure creates a lipophilicity that causes the pigments both to retard lipid peroxidation and stabilize lipid–protein structures like cell membranes. The polyene chain is mainly responsible for the chemical reactivity of carotenoids toward oxidizing agents and free radicals. Zeaxanthin is the stereoisomer of lutein (identical chemical formulas, but slightly different in their structure). Lutein and zeaxanthin differ merely in the placement of a single C=C double bond but possess noticeable biological functions (). The hydroxyl groups appear to control the biological function of these two xanthophylls. Lutein and zeaxanthin constitute the main carotenoids in egg yolk (Widomska & Subczynski, Citation2014). Other xanthophyll carotenoids found in egg yolk include β-cryptoxanthin, cis isomers of lutein and zeaxanthin, and synthetic xanthophylls (Nimalaratne, Lopes-Lutz, Schieber, & Wu, Citation2012; Schlatterer & Breithaupt, Citation2006). shows the chemical structure of xanthophylls found in egg yolk, and these xanthophylls include all-trans lutein, all-trans zeaxanthin, all-trans canthaxanthin (synthetic xanthophyll), all-trans β-apo-8′-carotenoic acid ethyl ester (synthetic xanthophyll), and their cis isomers. All of these carotenoids deposited in the yolk are influenced by the hen’s diet (Nimalaratne et al., Citation2012).
Figure 1. Chemical structure of lutein and zeaxanthin.
Structure of lutein (single stranded rings). Structure of zeaxanthin (one double bond in each ring).
Figura 1. Estructura química de luteína y zeaxantina.
Estructura de luteína (anillos monocatenarios)Estructura de zeaxantina (un enlace doble en cada anillo)
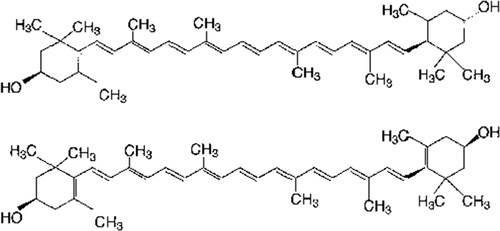
Figure 2. Structures of egg yolk carotenoids. Modified from Nimalaratne et al. (Citation2013).
Figura 2. Estructuras de carotenoides de yema de huevo. Modificado de Nimalaratne et al. (Citation2013).
Also, it is worth to mention here that xanthophyll carotenoids, once introduced in vivo (by dietary intake), are strongly influenced by other subcellular structures like proteins and membrane lipid. Structural features such as size, shape, and polarity are essential determinants of the ability of a carotenoid to fit correctly into its molecular environment to allow it to function. Carotenoids play an important role in modifying structure, properties, and stability of cell membranes, and thus affecting molecular processes associated with these membranes, leading to possible beneficial effects on human health (Britton, Citation1995).
Chemistry of lutein and zeaxanthin is important as these are the only two carotenoids identified in the human eye lens with protective function against age-related increases in lens density (Hammond, Wooten, & Snodderly, Citation1997).
3. Analysis of egg carotenoids
Analysis of lutein, zeaxanthin, and other carotenoids in eggs presents special challenges because of the high fat (~27%) and protein (~15%) contents in the yolk matrix, especially since they are susceptible to heat and light and may be degraded in the presence of lipid peroxides. Furthermore, xanthophyll may be present both in free form and as fatty acid esters, which may affect their polarity and hence their solubility (Nimalaratne, Wu, & Schieber, Citation2013). Most of the procedures reported in research studies for the analysis of egg yolk carotenoids rely on their extraction with organic solvents followed by a purification step to remove co-extracted lipids. Extraction of carotenoids from egg yolk samples for analysis using a single solvent system (Furusawa, Citation2011, Citation2013) or mixed solvent system followed by cleanup on mini-columns with a variety of absorbents (Brulc, Simonovska, Vovk, & Glavnik, Citation2013) is on record and successful. An excellent recovery of >99% was found when a ternary solvent system consisting of light petroleum ether, ethyl acetate, and methanol (1:1:1, volume/volume/volume) was used for the extraction of xanthophylls from egg yolk (Schlatterer & Breithaupt, Citation2006). Likewise, acetone, methanol, and 0.5 M triethylammonium acetate (14:5:1, volume/volume/volume) is also an efficient solvent system for the extraction of egg yolk carotenoids (Brulc et al., Citation2013). Extraction of lutein from egg yolk by ultrasound-assisted solvent extraction was more efficient than the single solvent method (Yue, Xu, Prinyawiwatkul, & King, Citation2006). Following extraction, carotenoids in egg yolk extracts can be separated and quantified using several analytical techniques. Research techniques for the fast determination or analysis of carotenoids continue to be reported by range of researchers. These techniques include high-performance liquid chromatography (HPLC), liquid chromatography–mass spectrometry (LC–MS), liquid chromatography–tandem mass spectrometry (LC–MS/MS), and ultra-high performance liquid chromatography (UHPLC) (Brulc et al., Citation2013; Kopec, Cooperstone, Cichon, & Schwartz, Citation2012; Machmudah & Goto, Citation2013; Ow, Salim, Noirel, Evans, & Wright, Citation2011; Rivera & Canela-Garayoa, Citation2012; Swartz, Citation2005; Wenzel, Seuss-Baum, & Schlich, Citation2010, Citation2011). Brief details and application of these techniques are given here.
The most commonly used technique has been and continues to be the HPLC for routine carotenoid analysis. Although a number of normal-phase columns have been reported, reverse-phase columns are the most widely used stationary phases for the analysis of these molecules. The most frequently used columns are C30 that provide high resolutions in the separation of carotenoids with similar structures compared to C18 columns (Strati, Sinanoglou, Kora, Miniadis-Meimaroglou, & Oreopoulou, Citation2012). The column operating temperature can also influence efficiency of separation with the most commonly used temperature range being 25–35°C (Brulc et al., Citation2013; Nimalaratne et al., Citation2013). The LC–MS technique has been employed to confirm and quantify the presence of lutein and zeaxanthin in egg yolks. The use of an isocratic elution has also been recognized in the separation of carotenoids present in egg yolks (Brulc et al., Citation2013). Mass spectrometry (LC–MS/MS) is efficient for carotenoid identification through the use of transitions for the detection of analytes through precursor and daughter ions. This approach is suitable for the identification of carotenoids with the same molecular mass but different fragmentation patterns (Rivera & Canela-Garayoa, Citation2012).
UHPLC is a relatively new technique, which has advantages over conventional HPLC in terms of an increase in resolution with narrower peaks, sensitivity, and speed of analysis (due to shorter retention times). UHPLC allows for a higher sample throughput and is more cost-effective. Also, UHPLC is capable of separating egg yolk carotenoids, including cis isomers of lutein and zeaxanthin, within less than 10 min, whereas approximately 80 min was required using conventional HPLC (Wenzel et al., Citation2011). Apart from laboratory-based analysis, recently a report appeared in print (Brulc et al., Citation2013) regarding the use of portable iCheck method (laboratory-independent conditions) to determine the total carotenoid of egg yolk (Islam & Schweigert, Citation2015). Advantage of this method is its easy operation with less time involved to analyze egg yolk carotenoid samples compared to above chromatographic techniques.
Over all, in broader perspectives (egg and others), methodologies for the analysis of carotenoids including lutein and zeaxanthin in eggs (Brulc et al., Citation2013; Nimalaratne et al., Citation2013), grains (Abdel-Aal, Young, Rabalski, Hucl, & Fregeau-Reid, Citation2007), juices (Meléndez-Martínez, Vicario, & Heredia, Citation2007), vegetables and fruit (Chen, Tai, & Chen, Citation2004; Huck, Popp, Scherz, & Bonn, Citation2000), infant formulas (Yuhas et al., Citation2011), and biological materials and fluids (Khachik, Bernstein, & Garland, Citation1997) have extensively been reported and successfully applied. Important dietary sources of carotenoids, other than hen’s egg, include fruits and vegetables like papayas mangoes, pumpkin carrots, spinach, corn, tomatoes, and others (Abdel-Aal et al., Citation2013). Epidemiological evidence indicated that a diet rich in fruits and vegetables could lower the risk of certain cancers, cardiovascular, and eye diseases (Milani, Basirnejad, Shahbazi, & Bolhassani, Citation2016; Wang et al., Citation2016). This may, possibly, be due to the affirmative role of ‘bioactive’ in controlling the cellular mechanisms causing carcinogenesis. Therefore, analytical techniques for the quantitative determination of carotenoids in complex sample matrices are important. Lutein amount was evaluated from range of fruits and vegetables by employing a technique referred to as alkaline hydrolysis extraction method coupled with HPLC analysis (Fratianni, Mignogna, Niro, & Panfili, Citation2015). This method is simple, precise, and accurate in retrieving carotenoids contents from fruit and vegetables. However, the bioavailability of carotenoids from these dietary sources may be low. This may be due to the physical state of the pigments, processing, and the presence of lipids, whereas carotenoids from egg yolk have demonstrated to have higher bioavailability (Handleman, Nightingale, Lichtenstein, Schaefer, & Blumberg, Citation1999). The average content of lutein and zeaxanthin in the yolk is ~200–300 μg; the lipid matrix allows for efficient uptake of these pigments. As such, hen’s egg can be considered an ideal carrier of biologically active carotenoids for human consumption. Also, moderate egg consumption is no longer associated with an increased risk of developing coronary heart diseases in healthy individuals.
4. Composition of egg carotenoids
Egg yolk is highly available and affordable source of lutein and zeaxanthin. This source can be considered an ideal carrier of biologically active carotenoids beneficial for, apart from human consumption, good eye health and vision in all age groups, and other health issues. However, the profile of carotenoids in egg yolk is highly dependent on the hens’ diet. This means that the type and amount of carotenoids in yolk can be manipulated through poultry feed handling. Similarly, different rearing systems produce eggs with distinct yolk carotenoid composition because of the differences in feed utilization (Schlatterer & Breithaupt, Citation2006).
Among corn products, only yellow cornmeal is common feed additives in hen’s feed for enriched eggs. As such, lutein and zeaxanthin contents contained in enriched raw egg (yolk + white) and egg yolk, from chickens raised on a well-defined feed, were recorded (Perry, Rasmussen, & Johnson, Citation2009). shows that on average, egg yolks contain fairly high amounts of lutein and zeaxanthin at concentrations of 1282 ± 182 and 640 ± 140 µg/100 g (n = 6), respectively (Nimalaratne et al., Citation2013). These amounts include all-trans and cis isomers of lutein and zeaxanthin. High concentrations of lutein (1774 μg/100 g) and zeaxanthin (1021 μg/100 g, n = 7) were reported in egg yolk of ecological husbandry (organic) which were higher than other husbandry systems (free range, barn, and caged) probably because hens of the ecological system had access to dark green leafy vegetation on their pastures, whereas the hens in the other systems did not (Schlatterer & Breithaupt, Citation2006). The β-cryptoxanthin was also present in eggs from the ecological husbandry but at low concentrations (83 ± 15 µg/100 g, n = 7). Likewise, another study revealed production of eggs with a very high level of lutein (4600 µg/100 g) and zeaxanthin (2435 µg/100 g) in yolk of eggs from chickens raised on a well-defined feed for which details are not provided because of proprietary restrictions (Kelly, Plat, Haenen, Kijlstra, & Berendschot, Citation2014). These values are more than 2 and 3 times higher than those obtained from ecological husbandry mentioned above. In the same perspective, research activities are on record to evaluate the ability of designer eggs enriched with certain nutrients (such as vitamin E, lutein, selenium, docosahexaenoic acid, etc.) to deliver protein, fats, and micronutrients to the human in visually acceptable form (Miranda et al., Citation2015; Surai, MacPherson, Speake, & Sparks, Citation2000). As such, designer egg, with altered nutrient composition, is considered as a new type of functional food.
Table 1. Lutein and zeaxanthin contents (µg/100 g of dry matter) in raw egg, egg yolk.
Tabla 1. Contenido de luteína y zeaxantina (µg/100 g de materia seca) en huevo crudo, yema de huevo.
5. Effect of cooking and processing on egg carotenoids
Eggs for human consumption are routinely cooked by boiling or frying either alone or with other ingredients to meet personal preferences. Cooking and processing may possibly cause some depletion in the contents of egg carotenoids because the highly reactive, electron-rich carotenoid molecule present in egg yolks suffers oxidation. The level of oxidation depends on the amount of carotenoid present, available oxygen, exposure to light, temperature, and the presence of enzymes, metals, and antioxidants. But, on the other hand, mechanical disruption or heat treatment causes the cell wall to soft or break and membrane, thereby, facilitates the release of health promoting components and as such increases their bioavailability. Processing conditions should therefore be optimized so that bioavailability is increased.
Research articles appeared in press selectively reviewed and briefly summarized the effect of processing on egg yolk (Nimalaratne et al., Citation2013). Lutein, zeaxanthin, and other carotenoids found in poultry feed are oxygenated and are transferred to eggs in that form. These carotenoids are highly susceptible to heat, light, and moisture and thus can undergo structural changes during cooking or processing (Schieber & Carle, Citation2005). Lutein, zeaxanthin, and canthaxanthin were reduced by cooking but to different extents (Nimalaratne et al., Citation2012, Citation2013). For example, all-trans lutein in egg yolk was the most affected with reductions of about 23%, 17%, and 19% for boiled, microwaved, and fried eggs, respectively. In view of the above mentioned properties of carotenoids, and considering that eggs constitute a matrix rich in lipids (Blesso, Citation2015), one would expect that significant isomerization of egg yolk xanthophylls may occur during storage and processing (Kalt, Citation2005; Rodriguez-Amaya, Citation1999, Citation2002). But according to recent investigations, into the stability of egg yolk xanthophylls during domestic cooking, it is revealed that the qualitative profile of carotenoid stereoisomers does not change upon heating (Nimalaratne et al., Citation2013). Boiling, frying, and microwave heating led to a significant decrease, especially in all-trans lutein and a concomitant slight increase in the 13 cis isomers of lutein and zeaxanthin, which however was less pronounced than expected. Total losses of xanthophylls ranged from 6% to 18% (Nimalaratne et al., Citation2013).
Also important is the oxidation mechanisms by which carotenoids are degraded, including pathways induced by heat, light, oxygen, acid, transition metal, or interactions with radical species (Boon et al., Citation2010). Free aromatic amino acids in egg yolk show antioxidant properties. Egg yolk contains considerable amount of antioxidants and is characterized to be tryptophan and tyrosine (Nimalaratne, Lopes-Lutz, Schieber, & Wu, Citation2011). Cooking may possibly reduce the antioxidant activities and the contents of aromatic amino acids. All cooking methods somehow reduced (p < 0.05) the antioxidant values of egg carotenoids (Nimalaratne et al., Citation2011). The polyene structure renders carotenoids susceptible to isomerization and degradation caused by conditions typically applied during processing. Under such conditions like high temperatures and UV light, presence of oxygen, and certain enzymes (mono- and dioxygenases, redox active metal ions), all-trans carotenoids may be converted to their cis isomers (Schieber & Carle, Citation2005) or oxidized and cleaved to apo-carotenoids (Carail & Caris-Veyrat, Citation2006; Fleischmann & Zorn, Citation2008). These conversions may be associated with a partial or complete loss of bioactivity, and/or the degradation products may show entirely different biological activities (Wang, Citation2004).
6. Feeding systems and egg carotenoids
Eggs are an inexpensive and low-calorie source of high-quality protein and other nutrients beneficial to human health. However, nutrition and husbandry system of the hens may significantly affect the quality of eggs. The profile of egg carotenoids is largely dependent on hen’s feed composition; therefore, it can vary among different types of eggs produced from conventional cages to either an enriched cage or a non-cage system (Karadas, Grammenidis, Surai, Acamovic, & Sparks, Citation2006; Nimalaratne et al., Citation2013; Schlatterer & Breithaupt, Citation2006). Beyond doubt, the chemical and nutrient composition of hen’s egg is well documented (Kovacs-Nolan, Phillips, & Mine, Citation2005; Li-Chan & Kim, Citation2008; Seuss-Baum, Citation2007). Researchers analyzed quality parameters of poultry from organic and conventional farms (Sherwin, Richards, & Nicol, Citation2010). Different rearing systems produce eggs with distinct yolk carotenoid composition because of the differences in feed utilization (Schlatterer & Breithaupt, Citation2006). Layers can be kept in different systems. The profile of carotenoids in egg yolk is highly dependent on the hens’ diet (based on the standardized poultry feed) as well as conducive management systems and or requirements, details of which are given as under:
6.1. Confinement
Confinement facilitates egg collection, hen capture, protection from predators and pests, and adverse climates. Confinement systems include on range in fields/paddocks where climates are warm enough, confined indoors in pens with access to range, confined totally indoors either in pens or cages with either single or multiple hens/cage. Houses must incorporate ventilation systems to provide fresh air and to maintain temperature and humidity produced by the chickens. Layers generate a great deal of body heat (Castellini, Mugnai, & Dal Bosco, Citation2002; Combes et al., Citation2003; Sherwin et al., Citation2010).
6.2. Management systems
Management systems have developed with minimum standards to meet the hen’s needs for health and safety (Commission of the European Communities (CEC), Citation1999). These usually include provision of water and feed, shelter and/or ventilation, artificial lighting, cleaning, and/or disinfection of facilities. These needs become more intensive and critical as the space per hen is reduced and production increases. During the past century, improved feeding and lighting systems have led to year round egg production which was supported by advanced confinement and management systems. Globally, a range of relevant management systems is applied (Sherwin et al., Citation2010). The specific type is determined largely by climatic and economic constraints. These systems have increased production efficiency and reduced labor.
6.3. Nutrition
Proper nutrition is critical for optimal production. Energy, protein, mineral, and vitamin requirements of laying hens are determined by maintenance, body weight, and level of egg production (Leeson, Citation2011). Ingredients are selected on availability, price, and, if available, nutrient bioavailability estimates, to minimize ration costs. Laying hens require higher levels of calcium and vitamins A, D, and choline than other chickens (Leeson, Citation2011).
6.4. Operation size
Layer operation size in developed countries has increased dramatically during the last century. Flocks of 100,000 laying hens are common with some exceeding 1 million (National Agricultural Statistics Service [NAAS], Citation2013). Large layer farms consist of several large houses of in-line and off-line types (United States Environmental Protection Agency [US-EPA], Citation2012). In in-line production systems, eggs from all houses are gathered and transferred to an adjacent plant for processing and refrigeration prior to shipping.
6.5. Housing
Most egg production is carried out using conventional cage systems, where layers live in cages and have limited mobility. Housing density and reduced space per hen have aroused concern about the hen’s welfare. The European Union Council Directive on welfare of laying hens, 1999/74/EC, required conventional laying cages to be phased out by 2012 (CEC, Citation1999). Traditional cages have been modified with enrichments and non-cage systems encouraged. Production costs of eggs from free-range hens versus caged hens may be increased by 50% or more (European Commission [EC], Citation2004; Sumner et al., Citation2011). Family poultry production systems are promoted in Ghana, Tanzania, and Zambia by the Food and Agriculture Organization of the United Nations and the International Egg Commission. Recommendations to improve management and production for these smaller operations are made available (Food and Agriculture Organization (FAO), Citation2012; Kumaresan et al., Citation2008). Housing facilities (conventional cages vs. enriched cages vs. pens) accounted for relatively small differences in egg production, mortality, and feed used per egg (Gerzilov, Datkova, Mihaylova, & Bozakova, Citation2012).
6.6. Hen’s organic eggs
Where climates permit and to meet consumer demands, ‘organic eggs’ are now produced by avoiding antibiotics and synthetic chemicals. Hens producing organic eggs must have access to the outdoors and cannot be housed in cages (Sherwin et al., Citation2010). The hen’s feed must be free of antibiotics and synthetic chemicals and must include grains from only crops certified as ‘organic.’ Land to produce such crops must have been free of ‘genetically modified’ crops and synthetic fertilizers for three or more years. Antibiotic use is permitted only for disease outbreaks (Cherian, Holsonbake, & Goeger, Citation2002; Sherwin et al., Citation2010).
In short, different systems vary (based on the standardized poultry as well as conductive management systems and or requirements) in how the egg-laying hens are housed, fed, and managed. Many studies have investigated how different housing systems and nutrition affect the quality of eggs and carotenoids (Mugnai et al., Citation2014). The composition of carotenoids in organic egg yolks differed from the nonorganic eggs, free range, and barn farms, as they contained higher concentrations of the natural xanthophylls of lutein and zeaxanthin and lower concentrations of the synthetic xanthophyll of canthaxanthin (van Ruth et al., Citation2011). Organic, free range, and barn eggs were clearly differentiated, by employing carotenoid profiling on perspective egg yolks. Experimental design included eggs from 12 organic, 12 free range, and 12 barn farms and subsequent analysis through the application of carotenoid HPLC – diode array detection profiling combined with k-nearest neighbor classification chemometrics. This analytical approach is worth to differentiate eggs from different husbandry systems (van Ruth et al., Citation2011). Overall, farming practices may influence the carotenoid composition of egg yolk.
7. Role of hen’s feed additives
The profile of egg carotenoids is largely dependent on hen’s feed composition; therefore, it can vary among different types of eggs (Karadas et al., Citation2006; Schlatterer & Breithaupt, Citation2006). In order to streamline and regulate the use of additives in the hen’s diet, feeds’ regulations were also introduced. The use of synthetic xanthophylls as feed additives has been permitted by the European Union, Canada, the United States, and other countries. The synthetic xanthophylls are obtained through chemical synthesis (Breithaupt, Citation2007) and are subject to regulatory limits which differ among countries (Nimalaratne et al., Citation2013). In the European Union, eight carotenoids are approved as additives in poultry feed. These are capsanthin (C40), β-cryptoxanthin (C40), lutein (C40), zeaxanthin (C40), β-apo-8′-carotenal (C30), β-apo-8′-carotenoic acid ethyl ester (C30), canthaxanthin (C40), and citranaxanthin (C33) (Becquet, Citation2003). In Canada, only β-apo-8′-carotenoic acid ethyl ester, lutein, and canthaxanthin are permitted according to the Canadian Feeds Regulation issued in 1983. The United States Food and Drug Administration sets limits for canthaxanthin as ≤30 mg/lb of solid or semisolid food or per pint of liquid food for general use and ≤4.41 mg/kg for broiler chicken feed (Abdel-Aal et al., Citation2007; Nimalaratne et al., Citation2013).
Naturally occurring xanthophylls from plant extracts like marigold (Tagetes erecta) or alfalfa (Medicago sativa) extracts and carotenoid-rich grains such as corn (Zea mays) and red pepper (Capsicum annuum) are also used to boost carotenoids in eggs and to improve egg yolk color (Breithaupt, Citation2007). Furthermore, just as different strains of corn have different levels of lutein and zeaxanthin, some chicken breeds deposit more lutein and zeaxanthin into their eggs than others (Pintea, Dulf, Bunea, Matea, & Andrei, Citation2012). These natural carotenoids can be included in the diets of hens of all housing systems, including the production of both organic and nonorganic eggs.
Naturally enriched eggs were made by increasing the levels of the xanthophylls lutein and zeaxanthin in the feed given to laying hens (Kelly et al., Citation2014; Nolan et al., Citation2016). Algae in laying hen diets can also influence carotenoid content of egg yolks. Laying hens fed five diets based on rapeseed/corn oils with or without microalgae (Nannochloropsis oculata) produced eggs that vary in carotenoid content and fatty acid composition of egg yolk (Fredriksson, Elwinger, & Pickova, Citation2006; Gładkowski et al., Citation2011). The microalgae were added to the feed as a source of n-3 long-chain polyunsaturated fatty acids and carotenoids. All diets were administered for 4 weeks to duplicate groups of five hens. The addition of algae to the diet increased the content of total carotenoids in egg yolk, e.g. from 970 µg/100 g in control to 3700 µg/100 g with 20% algae. It is now established that adding supplements to hen feed can increase egg nutritional value (Walker, Wang, Xin, & Dolde, Citation2012). For example, laying hens were fed palm tocols (better known as vitamin E) and algae astaxanthin (dark-red organic pigment) to improve nutritional quality of egg yolks with minimum changes in functional properties (Walker et al., Citation2012). The transfer of carotenoids into the egg yolks was also investigated using tomato peel and seed byproducts as a source of lycopene, which is a red carotenoid pigment (Knoblich, Anderson, & Latshaw, Citation2005). Tomato peel and seed byproducts added to hen diets at 75 g/kg resulted in a transfer of lycopene into egg yolks, i.e. from 0 to 90 µg/100 g. Approximately 0.1% and 0.7% of the lycopene in peel and seed byproducts, respectively, were transferred from the feed to the yolk. Because of the low efficiency in transfer of lycopene to the yolk of eggs compared with xanthophylls, lycopene appears to resemble carotene more than xanthophylls in its transfer to the yolk. It seems that carotenoids and other nutrients in hen diets are obviously transferred to the egg yolks, but their transfer efficiency is determined by their structure and interactions with other ingredients in the diet (Anton, Citation2007).
Over all, the diverse sources and types of carotenoids require special attention to produce efficient analyses and reliable results (Abdel-Aal et al., Citation2013). This means that profile of egg yolk carotenoids may vary significantly from country to country due to variable feed utilizations as per the feed regulations and the rearing system (Hargitai et al., Citation2006; Namitha & Negi, Citation2010; Nimalaratne et al., Citation2013). For example, in table eggs, from hens raised under traditional commercial conditions, six carotenoids are present at the greatest concentration. These include lutein, zeaxanthin, canthaxanthin, citranaxanthin, apo-carotene-ester, and cryptoxanthin. In contrast, organic eggs usually contain only lutein, zeaxanthin, and cryptoxanthin, as regulations do not allow synthetic carotenoid in the feed (Furusawa, Citation2011). Thus, to enhance the carotenoid content of egg yolk to a level that may be required, by humans, for the prevention of diseases and/or reduction in intensity (see Section 9), there is a continued need for research efforts in both the basic and the applied aspects of the subject being discussed, also to check or revise the feed regulation limitations, if needed or so required.
8. Bioavailability of egg carotenoids
Bioavailability refers to that portion of the nutrient or bioactive compound consumed that is released from the food matrix, absorbed, and used by the body. Lutein and zeaxanthin are bioavailable from range of food sources, which upon consumption gives efficient biological activities (Abdel-Aal et al., Citation2013; Fernández-García et al., Citation2012); but here, focus is only on egg carotenoids and benefits to human health. While the average content of lutein and zeaxanthin in the yolk is ~200–300 μg, the lipid matrix allows for efficient uptake of these pigments. To obtain maximum physiological benefits, lutein and zeaxanthin carotenoids must be absorbed and transported into the blood stream. In general, lutein and zeaxanthin carotenoids are lipophilic or hydrophobic which are soluble in fat and insoluble in aqueous media, the medium of human digestive system. Because of the hydroxyl groups, lutein and zeaxanthin are polar compounds compared with the hydrocarbon carotenoids (α-, β-carotene, and lycopene). Thus, a good understanding of carotenoid release, absorption, transportation, and accumulation in eye is essential to evaluate the benefits. In general, bioavailability of carotenoids is affected by a number of factors including food matrix, processing conditions, and fat content (van Het Hof, West, Weststrate, & Hautvast, Citation2000). Processing conditions (like heating, frying, etc.) which affects the bioavailability of egg yolk lutein and zeaxanthin should be optimized to minimize losses of these bioactive compounds with enhanced recovery for absorption.
The absorption of carotenoid released from egg consumption includes several steps: (1) dispersion in the gastric emulsion to be incorporated into lipid droplets, (2) followed by transfer to mixed micelles involving bile salts, biliary phospholipids (PL), dietary lipids, and others. Solubilized carotenoids are then absorbed by the intestinal cell for transportation into blood system. These steps may include simple diffusion, uptake by micelles, and receptor-mediated and other transporter (Abdel-Aal et al., Citation2013; Nagao, Citation2011). In humans, low- and high-density lipoproteins (HDLs) transport lutein and zeaxanthin via the systemic circulation to various tissues (Yeum & Russell, Citation2002). The highest concentration of carotenoids in micelles (i.e. solubilization) corresponds to greater absorption and transportation into plasma leading to possible prevention and/or slowing the progress of blindness and addresses other potential health concerns documented here in the following section.
9. Egg carotenoids for eye health and other health benefits
The health-promoting properties of carotenoid pigments are well documented (Fiedor & Burda, Citation2014). Here, we are focusing on hen’s egg (as a source) which is a unique and important carrier of bioactive carotenoids – lutein and zeaxanthin. These are lipid soluble in nature and are responsible for the orange-yellow color of the egg yolk. Bioavailability of lutein and zeaxanthin through egg consumption has been epidemiologically correlated with a lower risk for several diseases, details of which are given as under:
9.1. Age-related macular degeneration
In normal conditions, lutein and zeaxanthin accumulate in the macular region of the retina and are collectively referred to as macular pigment (MP). AMD is associated with a low level of MP in the eye retina. Only two carotenoids, namely lutein and zeaxanthin, are selectively accumulated in the human eye retina from blood plasma (Widomska & Subczynski, Citation2014). Because of its antioxidant and light-filtering properties, the MP may protect the retina and reduce the risk of developing AMD. As such, individuals who consume foods rich in lutein and zeaxanthin have a lower risk for AMD (Ma et al., Citation2012a; Seddon et al., Citation1994), higher blood levels of lutein and zeaxanthin (Handleman et al., Citation1999), and higher MP density (Hammond et al., Citation1997; Ma et al., Citation2012b).
Low level of lutein and zeaxanthin (with age) may cause AMD in humans, which in turn cause irreversible blindness. AMD has been a leading cause of irreversible blindness in the United States (Klein, Klein, Jensen, & Meuer, Citation1997). As human eye lens’ density increases (with age), the MP density decreases (Hammond et al., Citation1997). As such, researchers have studied and reviewed carotenoid-based visual cues and roles of carotenoids in human vision, and how the vision loss may be avoided or lessened by relevant nutritional input, especially dietary intake of lutein and zeaxanthin (Widomska, Zareba, & Subczynski, Citation2016). In nutshell dietary, provision of lutein and zeaxanthin may help to stop or slow down age-related increases in human eye lens’ density and, thereby, reducing the risks of AMD and cataracts (Demmig-Adams & Adams, Citation2013).
Predominant carotenoids of the MP in retina are lutein and zeaxanthin. Accurate assessment of the amount of MP, expressed as macular pigment optical density (MPOD), is therefore necessary to find out the role of carotenoids and their possible protective functions (de Kinkelder et al., Citation2011), as the hen’s egg yolk has comparable ingredients. Increasing egg consumption to six eggs per week or more may be an effective method to increase MPOD in humans, an indicator that the carotenoids from egg yolk may accumulate in the retina (Rong et al., Citation2013; Vishwanathan, Gendron, Goodrow-Kotyla, Wilson, & Nicolosi, Citation2010; Vishwanathan, Goodrow-Kotyla, Wooten, Wilson, & Nicolosi, Citation2009). Latest research findings strongly supported this concept by recognizing hen’s egg as a source of bioavailable lutein and zeaxanthin that are good for eye health and vision (Nimalaratne et al., Citation2015). Also, researchers investigated the effect of lutein- or zeaxanthin-enriched eggs or lutein-enriched egg-yolk-based buttermilk beverage on serum lutein and zeaxanthin concentrations and MPOD. Daily consumption of such intakes may possibly increases serum lutein and zeaxanthin levels that are comparable to a daily dose of 5 mg supplement (Kelly et al., Citation2014).
Overall, very little or no cure available for the treatment of AMD, but there are recommended dietary steps that may slow down the onset of these diseases by a variety of mechanisms including quenching of reactive oxygen species (ROS) that are responsible for such diseases (details follows in the proceeding section). It is well established that exposure to blue light (UV-B 280–315 nm) causes retinal degeneration (Kitchel, Citation2000). Any effort to minimize exposure to blue light would be an effort in the right direction to reduce the occurrence of cataract and AMD. In this perspective, both lutein and zeaxanthin act as a filter of harmful blue light in the eye and prevent the production of free radicals that are responsible for AMD (Hammond et al., Citation1997). This important function of lutein and zeaxanthin is possibly because of the presence of reactive oxygen in their structure with scavenging abilities (Fiedor & Burda, Citation2014). Also, lutein and zeaxanthin, with its strong antioxidative effects, can represent a viable solution in the complex treatment of glaucoma (Neacşu, Oprean, Curea, Tuchilă, & Trifu, Citation2003). In short, scientific evidence in support of the beneficial role of egg yolk bioactive in the prevention or reduction in intensity of AMD is on record (Chew et al., Citation2014; Nimalaratne et al., Citation2015), whereas unhealthy lifestyles can possibly increase AMD risk (Meyers et al., Citation2015).
9.2. CVDs, oxidative stress, Alzheimer’s, and cancer
In addition to being protector of vision, clinical research data also suggest that lutein and zeaxanthin may protect against CVDs (Andersen, Citation2015; Gammone, Riccioni, & D’Orazio, Citation2015; Shin et al., Citation2013), oxidative stress (Fiedor & Burda, Citation2014; Sen & Chakraborty, Citation2011), neurodegenerative disorders (Calabrese et al., Citation2010; Feart et al., Citation2016; Nataraj et al., Citation2015; Nolan et al., Citation2014, Citation2015), and possibly different types of cancer (Mares-Perlman et al., Citation2002). Over the years, hen eggs have acquired a bad reputation attributed to the high content of cholesterol mainly in yolks, which led to a serious decline in their consumption. For example, consumption of eggs in Canada went from 22.0 dozens/capita in 1980 to 17.1 dozens/capita in 1995. But a slow gradual increase in consumption started in 1996 due to a variety of factors including the introduction of designer eggs with omega-3 fatty acids and reached 20.5 dozens/capita in 2012 (Agriculture and Agri-Food Canada [AAFC], Citation2013). Egg intake may improve carotenoid status by increasing plasma HDL in adults with metabolic syndrome (Blesso et al., Citation2013). Egg yolk may represent an important food source to improve plasma carotenoid level in a population at high risk for CVD and type 2 diabetes (Shin et al., Citation2013). Based on preclinical studies, egg phosphatidylcholine and sphingomyelin appear to regulate cholesterol absorption and inflammation (Ballesteros et al., Citation2015; Blesso, Citation2015). PL are potential source of bioactive lipids present in chicken egg yolk and have widespread effects on pathways related to inflammation, cholesterol metabolism, and HDL function. Increased dietary cholesterol, lutein, and zeaxanthin consumed as egg yolks increase serum and retinal lutein and zeaxanthin without altering the serum status of the other carotenoids, tocopherol, and retinol (Vishwanathan et al., Citation2009). Also, eating four or more eggs/week was negatively correlated with serum cholesterol. Meta-analysis studies were conducted by a group of researchers (Shin et al., Citation2013) to determine relation of egg consumption versus risk of CVD, diabetes and observed no association or risks, with the exception of familial hypercholesterolemia subjects (Ruxton, Citation2010). Thus, it is important to look at eggs as more than a cholesterol-delivery system. Eggs are an inexpensive and low-calorie source of high-quality protein and other nutrients (Herron et al., Citation2006). In addition, the lipid matrix of the egg yolk enhances the bioavailability of valuable carotenoid pigments, including lutein and zeaxanthin without having any detrimental effects on lipoprotein or glucose metabolism (Ballesteros et al., Citation2015; Blesso, Citation2015).
The harmful effect of free radicals causing potential biological injury is termed oxidative stress. When free radicals are generated in vivo, many antioxidants act in defending from oxidative stress (Halliwell & Gutteridge, Citation1999). Excessive free radicals and ROS, such as the superoxide anion (O2−), hydroxyl radical (OH−), and the peroxy radical (ROO−), react with vital biomolecules (like lipids, proteins). It is increasingly thought that dietary intake of healthy food with antioxidant activity provides potential benefits in reducing the risk of some chronic diseases by maintaining redox homeostasis (Lee, Koo, & Min, Citation2004; Sen & Chakraborty, Citation2011).
Clinical studies are on record about the association of ROS with many age-related degenerative diseases, including atherosclerosis, vasospasms, cancers, trauma, stroke, asthma, hyperoxia, arthritis, heart attack, age pigments, dermatitis, cataractogenesis, retinal damage, hepatitis, liver injury, and periodontis (Calabrese et al., Citation2010; Cohen, Kristal, & Stanford, Citation2000; Packer, Weber, & Rimbach, Citation2001; Sen & Chakraborty, Citation2011). ROS also have been known to induce apoptosis of cells (Simon, Haj-Yehia, & Levi-Schaffer, Citation2000). Recently, a review article appeared in print thoroughly discussed the role of egg yolk carotenoids as an antioxidants exerting protective effects against oxidative damage (Nimalaratne & Wu, Citation2015). Molecular mechanisms are involved in the singlet oxygen and radical scavenging activity of lutein and zeaxanthin and as such exert beneficial effects in terms of decreasing or slowing down the light-induced oxidative stress in eye macular or AMD (Böhm, Edge, & Truscott, Citation2012; Krinsky, Landrum, & Bone, Citation2003; Li, Ahmed, & Bernstein, Citation2010). The predominant carotenoid in the fovea of the retina, zeaxanthin, scavenged hydroxyl radicals more effectively than the other retinal carotenoid, lutein (Trevithick-Sutton, Foote, Collins, & Trevithick, Citation2006). Functional benefits of lutein and zeaxanthin, as an antioxidants, were exhibited in another study where preincubation of human lens’ epithelial cells, with lutein, zeaxanthin, and α-tocopherol, dramatically reduced the levels of H2O2-induced protein carbonyl, malondialdehyde, and DNA damage (Gao et al., Citation2011). Also, lutein and zeaxanthin can scavenge peroxynitrite which may play a role in LDL protection against oxidative damage (Panasenko, Sharov, Briviba, & Sies, Citation2000).
Oxidative stress may possibly induce neuronal damage, modulate intracellular signaling, and eventually lead to neuronal death by apoptosis (Calabrese et al., Citation2010). Experimental evidence recorded lutein as the ‘enhancer’ of antioxidant defense against neuronal damages during diabetic retinopathy, ischemia, and Alzheimer’s (Nataraj et al., Citation2015). These properties of lutein may possibly cause decline in mitochondrial dysfunction and apoptotic death, indicating importance of lutein in treating Alzheimer’s. Homocysteine (Hcy), a sulfur containing nonprotein amino acid naturally present in the plasma, is implicated as a risk factor for numerous diseases owing largely to its free radical generating potency (Bonetti, Brombo, & Zuliani, Citation2016; Bukharaeva, Shakirzyanova, Khuzakhmetova, Sitdikova, & Giniatullin, Citation2015; Kamat, Vacek, Kalani, & Tyagi, Citation2015; Paul & Borah, Citation2015; Sharma, Kumar, Dar, & Singh, Citation2015). Also, epidemiological studies have found associations between high serum level of Hcy and Alzheimer’s disease (AD) progression that eventually leads to vascular dementia (VaD). After AD, the VaD is the second most common cause of dementia in people older than 65 (Kamat et al., Citation2015; Sharma et al., Citation2015). Emerging evidence indicates that higher concentrations of lutein in respect to plasma lipids may moderately decrease the risk of dementia and AD in an elderly community dwellers (Feart et al., Citation2016). AD patients exhibit significantly less MP and poorer vision [possibly due to lower serum concentrations of lutein and zeaxanthin (Nolan et al., Citation2014)]. Supplementation with the lutein and zeaxanthin carotenoids benefits patients with AD, in terms of not only clinically meaningful improvements in visual function but also cognitive function is triggered as merited (Nolan et al., Citation2014, Citation2015; Renzi, Dengler, Puente, Miller, & Hammond, Citation2014).
Cancer is one of the leading causes of mortality and disability worldwide. Bioactive components in dietary food or food-derived peptides provide an essential link in health maintenance, promotion, and prevention of chronic diseases, such as cancer (Hernández-Ledesma & Hsieh, Citation2015). Anticancer effect of dietary circulating carotenoids is surfacing (Milani et al., Citation2016; Wang et al., Citation2016; Yan et al., Citation2016), where bioactive components present in food can simultaneously modulate more than one potential cellular mechanisms. These mechanisms include apoptosis, antioxidant, anti-inflammation as well as the modulation of multiple molecular events causing carcinogenesis. Range of research studies indicates that the serum carotenoids, including lutein and zeaxanthin, are inversely associated with breast cancer risk among women (Eliassen et al., Citation2013; Yan et al., Citation2016). Similar inverse associations are on record between serum concentrations of zeaxanthin and other carotenoids and colorectal neoplasm (Okuyama et al., Citation2014). Likewise, associated with lutein plus zeaxanthin intake, research studies recorded decline in the rate of oral and pharyngeal cancer (18%) and laryngeal cancer (17%) (Leoncini et al., Citation2015), whereas other researchers reported inconsistent results between lutein/zeaxanthin intake and colorectal cancer risk (Lu et al., Citation2015) and breast cancer risk (Sisti et al., Citation2015). Overall number of preclinical and observational research studies regarding the role of lutein and zeaxanthin in prevention or reducing the intensity of different cancers continues to evolve from basic research as well as from human studies. These studies directed to bioavailability, metabolism, and dose–response relationships with intermediary biomarkers and clinical outcomes to determine and verify the role of lutein and zeaxanthin in controlling tumor growth in humans (Bertone et al., Citation2001; Boeke et al., Citation2014; Chew & Park, Citation2004; Cho et al., Citation2003; de Munter, Maasland, van den Brandt, Kremer, & Schouten, Citation2015; Fung et al., Citation2003; Gann et al., Citation1999; Ho et al., Citation2015; Jeurnink et al., Citation2015; Maggio et al., Citation2015; Niclis, Díaz Mdel, Eynard, Román, & La Vecchia, Citation2012; Nkondjock & Ghadirian, Citation2004; Silvera, Jain, Howe, Miller, & Rohan, Citation2006; Wang et al., Citation2014; Yuan, Stam, Arakawa, Lee, & Yu, Citation2003; Zhang et al., Citation1999). Although scientific evidence in support of the beneficial role of egg yolk carotenoids in prevention or reducing the intensity of AMD and CVD and neurodegeneration are substantial, research findings about the role of egg carotenoids on different cancers are inconclusive or inconsistent and warranted further research studies, meta-analyzes, to confirm the advantageous effect of egg carotenoids. It is only through such studies the possible affirmative role of egg carotenoids will be enhanced or confirmed leading to formulate strategies for the prevention, treatment, and management of cancerous diseases.
Taken together, egg yolk carotenoids are hypothesized to enhance antioxidant activity and provide potential benefits in reducing the risk of some chronic diseases. Egg consumers had considerably greater nutrient density contributing (apart from MP) vitamin A, E, folate, and B12 (Song & Kerver, Citation2000). Tocotrienols and tocopherols (i.e. tocos) are fat-soluble vitamins and are generically regarded as vitamin E, for which the antioxidant properties are well studied. Vitamin E is thought to prevent atherosclerosis, protect against coronary disease, and prevent neurons from oxidative stress and the development of AD and possibly cancers (Aggarwal, Sundaram, Prasad, & Kannappan, Citation2010; Rong et al., Citation2013).
9.3. Lutein and zeaxanthin supplementation
Alongside egg consumption as functional food, the use of lutein and zeaxanthin supplementation is also tested. Purified supplements of carotenoids can help reduce the possibility of getting eye diseases (AMD and cataracts). As such, the prevalence of lutein and zeaxanthin in supplements is increasing (Mares, Citation2016). Intake of supplement dosage 10 mg/day of lutein and 2 mg/day for zeaxanthin is considered as recommended level for reducing the risk of chronic eye diseases in humans (AOA, Citation2012). Early functional abnormalities of the central retina in the early AMD patients could be improved by lutein and zeaxanthin supplementation. MPOD may possibly be elevated due to lutein and zeaxanthin supplement intake (Ma et al., Citation2012b). Also, according to meta-analysis of longitudinal studies (Ma et al., Citation2012b), lutein and zeaxanthin affect positively in the case of late AMD but not early AMD. The early or dry AMD was defined by the presence of drusen pigment abnormalities in retina pigment epithelium (RPE) or both, whereas the late or wet AMD includes neovascular AMD and geographic atrophy by the presence of choroidal neovascularization, detachment of RPE, or geographic atrophy. In short, lutein and zeaxanthin are considered as appropriate supplement in controlling AMD (AOA, Citation2012; Ma et al., Citation2012b), duly backed by the AREDS2 (Age-Related Eye Disease Study 2) (Chew et al., Citation2014). This study evaluated the significance of replacing β-carotene with lutein/zeaxanthin in the AREDS formulation (Chew et al., Citation2014) because of the demonstrated risk for lung cancer from β-carotene in smokers and former smokers (Tanvetyanon & Bepler, Citation2008). Overall, consensual evidence suggests that lutein/zeaxanthin could be more appropriate supplement than β-carotene in curing age-related eye diseases (Chew et al., Citation2014).
10. Conclusions
The incidence of age-related diseases will continue as our population ages. By the year 2020, the number of people older than 60 years is expected to top 1 billion. The need to identify risk factors for disease must be evaluated along with diet and lifestyle factors that promote healthy aging. According to recent report (Centers for Disease Control and Prevention (CDC), Citation2014), the estimated number of blind and visually impaired people will be doubled by 2030. These estimates are in line with other regional and international authorities (Owen et al., Citation2012; The International Agency for the Prevention of Blindness, Citation2014). Thus, it is crucial to minimize this expected increase in morbidity and to diminish its associated costs.
Diet rich in lutein and zeaxanthin carotenoids, especially egg yolks, has been epidemiologically correlated with a lower risk for several diseases, especially the incidences of eye diseases, CVD, and neuronal damage. Periodical usage also helps in immunomodulation as antioxidants, evidenced by the high disappearance rate of carotenoids from the blood stream during immune stress periods. This, as antioxidant, in turn helps to reduce the incidences of related health concerns.
New marketing strategies should highlight eggs as an exceptional source of highly bioavailable lutein and zeaxanthin emphasizing their importance in human health. Meanwhile, the consumer needs to be better informed of the high quality attributes of eggs so as to repair the bad reputation of the past which focused on the high content of saturated fat and cholesterol in eggs. The public needs to be continuously reminded that science-based medical studies conclusively showed that consuming one eggs per day will not increase blood cholesterol in healthy humans, rather egg nutrients act as the ‘enhancer’ of antioxidant defense against range of diseases.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Abdel-Aal, E.-S.M., Young, J.C., Rabalski, I., Hucl, P., & Fregeau-Reid, J. (2007). Identification and quantification of seed carotenoids in selected wheat species. Journal of Agricultural and Food Chemistry, 55, 787–794. [PubMed]. doi:10.1021/jf062764p
- Abdel-Aal, E.-S., Akhtar, H., Zaheer, K., & Ali, R. (2013). Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health. Nutrients, 5, 1169–1185. doi:10.3390/nu5041169
- Agarwal, M., Parameswari, R.P., Vasanthi, H.R., & Das, D.K. (2012). Dynamic action of carotenoids in cardioprotection and maintenance of cardiac health. Molecules, 17, 4755–4769. doi:10.3390/molecules17044755
- Aggarwal, B.B., Sundaram, C., Prasad, S., & Kannappan, R. (2010). Tocotrienols, the vitamin E of the 21st century: Its potential against cancer and other chronic diseases. Biochemical Pharmacology, 80, 1613–1631. doi:10.1016/j.bcp.2010.07.043
- Agriculture and Agri-Food Canada (AAFC). (2013). Canada’s poultry and egg industry profile. Retrieved from http://www.agr.gc.ca/eng/industry-markets-and-trade/statistics-and-market-information/by-product-sector/poultry-and-eggs/poultry-and-egg-market-information-canadian-industry/industry-profile/?id=1384971854389
- American Optometric Association: Diet, Nutrition and Eye Health. (2012). Retrieved from http://www.aoa.org/patients-and-public/caring-for-your-vision/diet-and-nutrition/lutein?sso=y
- Andersen, C.J. (2015). Bioactive egg components and inflammation. Nutrients, 7, 7889–7913. doi:10.3390/nu7095372
- Anton, M. (2007). Composition and structure of hen egg yolk. In R. Huopalahti, R. López-Fandiño, M. Anton, & R. Schade (Eds.), Bioactive egg compounds (pp. 1−5). Berlin, NY: Springer.
- Ballesteros, M.N., Valenzuela, F., Robles, A.E., Artalejo, E., Aguilar, D., Andersen, C.J., … Fernandez, M.L. (2015). One egg per day improves inflammation when compared to an oatmeal-based breakfast without increasing other cardiometabolic risk factors in diabetic patients. Nutrients, 7, 3449–3463. doi:10.3390/nu7053449
- Becquet, P. (2003). EU assessment of enterococci as feed additives. International Journal of Food Microbiology, 88, 247–254. [PubMed]. doi:10.1016/S0168-1605(03)00187-9
- Bertone, E.R., Hankinson, S.E., Newcomb, P.A., Rosner, B., Willett, W.C., Stampfer, M.J., & Egan, K.M. (2001). A population-based case-control study of carotenoid and vitamin A intake and ovarian cancer (United States). Cancer Causes Control, 12, 83–90. [PubMed]. doi:10.1023/A:1008985015927
- Blesso, C.N. (2015). Egg phospholipids and cardiovascular health. Nutrients, 7, 2731–2747. doi:10.3390/nu7042731
- Blesso, C.N., Andersen, C.J., Bolling, B.W., & Fernandez, M.L. (2013). Egg intake improves carotenoid status by increasing plasma HDL, cholesterol in adults with metabolic syndrome. Food & Function, 4, 213–221. doi:10.1039/c2fo30154g
- Boeke, C.E., Tamimi, R.M., Berkey, C.S., Colditz, G.A., Eliassen, A.H., Malspeis, S., … Frazier, A.L. (2014). Adolescent carotenoid intake and benign breast disease. Pediatrics, 133, e1292–e1298. doi:10.1542/peds.2013-3844
- Böhm, F., Edge, R., & Truscott, G. (2012). Interactions of dietary carotenoids with activated (singlet) oxygen and free radicals: Potential effects for human health. Molecular Nutrition & Food Research, 56, 205–216. doi:10.1002/mnfr.201100222
- Bonetti, F., Brombo, G., & Zuliani, G. (2016). The relationship between hyperhomocysteinemia and neurodegeneration. Neurodegenerative Disease Management, 6, 133–145. doi:10.2217/nmt-2015-0008
- Boon, C.S., McClements, D.J., Weiss, J., & Decker, E.A. (2010). Factors influencing the chemical stability of carotenoids in foods. Critical Reviews in Food Science and Nutrition, 50, 515–532. doi:10.1080/10408390802565889
- Breithaupt, D.E. (2007). Modern application of xanthophylls in animal feeding – a review. Trends in Food Science & Technology, 18, 501–506. doi:10.1016/j.tifs.2007.04.009
- Britton, G. (1995). Structure and properties of carotenoids in relation to function. FASEB Journal, 9, 1551–1558.
- Brulc, L., Simonovska, B., Vovk, I., & Glavnik, V. (2013). Determination of egg yolk xanthophylls by isocratic high performance liquid chromatography. Journal of Chromatography A, 1318, 134–141. doi:10.1016/j.chroma.2013.09.074
- Bukharaeva, E., Shakirzyanova, A., Khuzakhmetova, V., Sitdikova, G., & Giniatullin, R. (2015). Homocysteine aggravates ROS-induced depression of transmitter release from motor nerve terminals: Potential mechanism of peripheral impairment in motor neuron diseases associated with hyperhomocysteinemia. Frontiers in Cellular Neuroscience, 9, 391. doi:10.3389/fncel.2015.00391
- Calabrese, V., Cornelius, C., Mancuso, C., Lentile, R., Stella, A.M., & Butterfield, D.A. (2010). Redox homeostasis and cellular stress response in aging and neurodegeneration. Methods in Molecular Biology, 610, 285–308. doi:10.1007/978-1-60327-029-8_17
- Carail, M., & Caris-Veyrat, C. (2006). Carotenoid oxidation products: From villain to saviour? Pure and Applied Chemistry, 78, 1493–1503. doi:10.1351/pac200678081493
- Castellini, C., Mugnai, C., & Dal Bosco, A. (2002). Effect of organic production system on broiler carcass and meat quality. Meat Science, 60, 219–225. doi:10.1016/S0309-1740(01)00124-3
- Centers for Disease Control and Prevention (CDC). (2014). Improving the nation’s vision health: A coordinated public health approach. Atlanta, GA. Retrieved from www.cdc.gov/visionhealth/publications/vhi_report.htm
- Chen, J.P., Tai, C.Y., & Chen, B.H. (2004). Improved liquid chromatographic method for determination of carotenoids in Taiwanese mango (Mangifera indica L.). Journal of Chromatography A, 1054, 261–268. doi:10.1016/S0021-9673(04)01406-2
- Cherian, G., Holsonbake, T.B., & Goeger, M.P. (2002). Fatty acid composition and egg components of specialty eggs. Poultry Science, 81, 30–33. doi:10.1093/ps/81.1.30
- Chew, B.P., & Park, J.S. (2004). Carotenoid action on the immune response. The Journal of Nutrition, 134, 257S–261S. [PubMed].
- Chew, E.Y., Clemons, T.E., Sangiovanni, J.P., Danis, R.P., Ferris, F.L., Elman, M.J., … Sperduto, R.D. (2014). Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmology, 132, 142–149. doi:10.1001/jamaophthalmol.2013.7376
- Cho, E., Spiegelman, D., Hunter, D.J., Chen, W.Y., Zhang, S.M., Colditz, G.A., & Willett, W.C. (2003). Premenopausal intakes of vitamins A, C, and E, folate, and carotenoids, and risk of breast cancer. Cancer Epidemiology, Biomarkers & Prevention, 12, 713–720. [PubMed].
- Cohen, J.H., Kristal, A.R., & Stanford, J.L. (2000). Fruit and vegetable intakes and prostate cancer risk. JNCI Journal of the National Cancer Institute, 92, 61–68. [PubMed]. doi:10.1093/jnci/92.1.61
- Combes, S., Lebas, F., Lebreton, L., Martin, T., Jehl, N., Cauquil, L., … Corboeuf, M.A. (2003). Comparison lapin « Bio »/lapin standard: Caractéristique des carcasses et composition chimique de 6 muscles de la cuisse. Proc. 10èmes Journées de la Recherche Cunicole, Paris, pp. 133–136.
- Commission of the European Communities (CEC). (1999). Council Directive 1999/74/EC laying down minimum standards for the protection of laying hens. Official Journal of the European Communities, L 203, 53–57.
- de Kinkelder, R., van der Veen, R.L.P., Verbaak, F.D., Faber, D.J., van Leeuwen, T.G., & Berendschot, T.T.J.M. (2011). Macular pigment optical density measurements: Evaluation of a device using heterochromatic flicker photometry. Eye (Lond), 25, 105–112. doi:10.1038/eye.2010.164
- de Munter, L., Maasland, D.H., van den Brandt, P.A., Kremer, B., & Schouten, L.J. (2015). Vitamin and carotenoid intake and risk of head-neck cancer subtypes in the Netherlands Cohort Study. The American Journal of Clinical Nutrition, 102, 420–432. doi:10.3945/ajcn.114.106096
- Demmig-Adams, B., & Adams, B.R. (2013). Eye nutrition in context: Mechanisms, implementation, and future directions. Nutrients, 5, 2483–2501. doi:10.3390/nu5072483
- Eliassen, A.H., Hendrickson, S.J., Brinton, L.A., Buring, J.E., Campos, H., Dai, Q., … Hankinson, S.E. (2013). Circulating carotenoids and risk of breast cancer: Pooled analysis of eight prospective studies. Journal of the National Cancer Institute, 104, 1905–1916. doi:10.1093/jnci/djs461
- European Commission (EC). (2004). Study on the socio-economic implications of the various systems to keep laying hens. Final Report for the European Commission, submitted by Agra CEAS Consulting Ltd., 2120/CC/December 2004.
- FAO (Food and Agriculture Organization). (2012). World egg day 2012. Retrieved from http://www.fao.org/ag/againfo/home/en/news_archive/2012_World_Egg_Day_2012.html
- Feart, C., Letenneur, L., Helmer, C., Samieri, C., Schalch, W., Etheve, S., … Barberger-Gateau, P. (2016). Plasma carotenoids are inversely associated with dementia risk in an elderly French cohort. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 71, 683–688. doi:10.1093/gerona/glv135
- Fernández-García, E., Carvajal-Lérida, I., Jarén-Galán, M., Garrido- Fernández, J., Pérez-Gálvez, A., & Hornero-Méndez, D. (2012). Carotenoids bioavailability from foods: From plant pigments to efficient biological activities. Food Research International, 46, 438–450. doi:10.1016/j.foodres.2011.06.007
- Fiedor, J., & Burda, K. (2014). Potential role of carotenoids as antioxidants in human health and disease. Nutrients, 6, 466–488. doi:10.3390/nu6020466
- Fleischmann, P., & Zorn, H. (2008). Enzymic pathways for formation of carotenoid cleavage products. Carotenoids, 4, 341–366. doi:10.1007/978-3-7643-7499-0_17
- Fratianni, A., Mignogna, R., Niro, S., & Panfili, G. (2015). Determination of lutein from fruit and vegetables through an Alkaline Hydrolysis Extraction Method and HPLC analysis. Journal of Food Science, 80, C2686–C2691. doi:10.1111/1750-3841.13122
- Fredriksson, S., Elwinger, K., & Pickova, J. (2006). Fatty acid and carotenoid composition of egg yolk as an effect of microalgae addition to feed formula for laying hens. Food Chemistry, 99, 530–537. doi:10.1016/j.foodchem.2005.08.018
- Fung, T.T., Spiegelman, D., Egan, K.M., Giovannucci, E., Hunter, D.J., & Willett, W.C. (2003). Vitamin and carotenoid intake and risk of squamous cell carcinoma of the skin. International Journal of Cancer, 103, 110–115. doi:10.1002/ijc.10798
- Furusawa, N. (2011). A simple and small-scale sample preparation technique to determine canthaxanthin in hen egg yolk. Food Chemistry, 124, 1643–1646. doi:10.1016/j.foodchem.2010.08.017
- Furusawa, N. (2013). An easy and small-scale sample preparation technique followed by HPLC-PDA analysis for determining canthaxanthin in chicken liver, fat, and egg yolk. Chemistry Journal, 3, 112–116. [PubMed].
- Gammone, M.A., Riccioni, G., & D’Orazio, N. (2015). Carotenoids: Potential allies of cardiovascular health? Food & Nutrition Research, 59, 26762. doi:10.3402/fnr.v59.26762
- Gann, P.H., Ma, J., Giovannucci, E., Willett, W., Sacks, F.M., Hennekens, C.H., & Stampfer, M.J. (1999). Lower prostate cancer risk in men with elevated plasma lycopene levels: Results of a prospective analysis. Cancer Research, 59, 1225–1230. PubMed.
- Gao, S., Qin, T., Liu, Z., Caceres, M.A., Ronchi, C.F., Chen, C.Y.O., … Liu, Y. (2011). Lutein and zeaxanthin supplementation reduces H2O2-induced oxidative damage in human lens epithelial cells. Molecular Vision, 17, 3180–3190. [PubMed].
- Gerzilov, V., Datkova, V., Mihaylova, S., & Bozakova, N. (2012). Effect of poultry housing systems on egg production. Bulgarian Journal of Agricultural Science, 18, 953–957.
- Gładkowski, W., Kiełbowicz, G., Chojnacka, A., Gil, M., Trziszka, T., Dobrzański, Z., & Wawrzeńczyk, C. (2011). Fatty acid composition of egg yolk phospholipid fractions following feed supplementation of Lohmann Brown hens with humic-fat preparations. Food Chemistry, 126, 1013–1018. doi:10.1016/j.foodchem.2010.11.112
- Goodrow, E.F., Wilson, T.A., Houde, S.C., Vishwanathan, R., Scollin, P.A., Handelman, G., & Nicolosi, R.J. (2006). Consumption of one egg per day increases serum lutein and zeaxanthin concentrations in older adults without altering serum lipid and lipoprotein cholesterol concentrations. Journal of Nutrition, 136, 2519–2524. [PubMed].
- Halliwell, B., & Gutteridge, J.M.C. (1999). Free radicals, other reactive species and disease. In B. Halliwell & J. Gutteridge (Eds.), Free radical in biology and medicine (pp. 617–783). Oxford: Oxford University Press.
- Hammond, B.R., Wooten, B.R., & Snodderly, D.M. (1997). Density of the human crystalline lens is related to the macular pigment carotenoids, lutein and zeaxanthin. Optometry and Vision Science, 74, 499–504. [PubMed]. doi:10.1097/00006324-199707000-00017
- Handleman, G.H., Nightingale, Z.D., Lichtenstein, A.H., Schaefer, E.J., & Blumberg, J.P. (1999). Lutein and zeaxnathin concentrations in plasm after dietary supplementation with egg yolk. American Journal of Clinical Nutrition, 70, 247–251. [PubMed].
- Hargitai, R., Matus, Z., Hegyi, G., Michl, G., Tóth, G., & Török, J. (2006). Antioxidants in the egg yolk of a wild passerine: Differences between breeding seasons. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 143, 145–152. doi:10.1016/j.cbpb.2005.11.001
- Hernández-Ledesma, B., & Hsieh, C.-C. (2015). Chemopreventive role of food-derived proteins and peptides: A review. Critical Reviews in Food Science and Nutrition, 2015 Nov 13:0. [Epub ahead of print]. doi:10.1080/10408398.2015.1057632
- Herron, K.L., McGrane, M.M., Waters, D., Lofgren, I.E., Clark, R.M., Ordovas, J.M., & Fernandez, M.L. (2006). The ABCG5 polymorphism contributes to individual responses to dietary cholesterol and carotenoids in eggs. Journal of Nutrition, 136, 1161–1165. [PubMed].
- Ho, W.J., Simon, M.S., Yildiz, V.O., Shikany, J.M., Kato, I., Beebe-Dimmer, J.L., … Bock, C.H. (2015). Antioxidant micronutrients and the risk of renal cell carcinoma in the Women’s Health Initiative cohort. Cancer, 121, 580–588. doi:10.1002/cncr.29091
- Huck, C.W., Popp, M., Scherz, H., & Bonn, G.K. (2000). Development and evaluation of a new method for the determination of the carotenoid content in selected vegetables by HPLC and HPLC-MS-MS. Journal of Chromatographic Science, 38, 441–449. [PubMed]. doi:10.1093/chromsci/38.10.441
- Islam, K.M., & Schweigert, F.J. (2015). Comparison of three-spectrophotometric methods for analysis of egg yolk carotenoids. Food Chemistry, 172, 233–237. doi:10.1016/j.foodchem.2014.09.045
- Jeurnink, S.M., Ros, M.M., Leenders, M., van Duijnhoven, F.J., Siersema, P.D., Jansen, E.H., … Bueno-de-Mesquita, H.B. (2015). Plasma carotenoids, vitamin C, retinol and tocopherols levels and pancreatic cancer risk within the European Prospective Investigation into Cancer and Nutrition: A nested case-control study: plasma micronutrients and pancreatic cancer risk. International Journal of Cancer, 136, E665–E676. doi:10.1002/ijc.29175
- Kalt, W. (2005). Effects of production and processing factors on major fruit and vegetable antioxidants. Journal of Food Science, 70, R11–R19. doi:10.1111/j.1365-2621.2005.tb09053.x
- Kamat, P.K., Vacek, J.C., Kalani, A., & Tyagi, N. (2015). Homocysteine induced cerebrovascular dysfunction: A link to Alzheimer’s disease etiology. The Open Neurology Journal, 9, 9–14. doi:10.2174/1874205X01509010009
- Karadas, F., Grammenidis, E., Surai, P.F., Acamovic, T., & Sparks, N.H. (2006). Effects of carotenoids from lucerne, marigold and tomato on egg yolk pigmentation and carotenoid composition. British Poultry Science, 47, 561–566. doi:10.1080/00071660600962976
- Kelly, E.R., Plat, J., Haenen, G.R.M.M., Kijlstra, A., & Berendschot, T.T.J.M. (2014). The effect of modified eggs and an egg-yolk based beverage on serum lutein and zeaxanthin concentrations and macular pigment optical density: Results from a randomized trial. Plos One, 9, e92659. doi:10.1371/journal.pone.0092659
- Khachik, F., Bernstein, P.S., & Garland, D.L. (1997). Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Investigative Ophthalmology & Visual Science, 38, 1802–1811. [PubMed].
- Kitchel, E. (2000). The effects of blue light on ocular health. Journal of Visual Impairment and Blindness, 94, 399–403.
- Klein, R., Klein, B.E., Jensen, S.C., & Meuer, S.M. (1997). The five-year incidence and progression of age-related macular degeneration. Ophthalmology, 104, 7–21. [PubMed]. doi:10.1016/S0161-6420(97)30368-6
- Knoblich, M., Anderson, B., & Latshaw, D. (2005). Analyses of tomato peel and seed byproducts and their use as a source of carotenoids. Journal of the Science of Food and Agriculture, 85, 1166–1170. doi:10.1002/jsfa.2091
- Kopec, R.E., Cooperstone, J.L., Cichon, M.J., & Schwartz, S.J. (2012). Analysis methods of carotenoids. In Z. Xu & L.R. Howard (Eds.), Analysis of antioxidant-rich phytochemicals. Oxford, UK: Wiley-Blackwell.
- Kovacs-Nolan, J., Phillips, M., & Mine, Y. (2005). Advances in the value of eggs and egg components for human health. Journal of Agricultural Food Chemistry, 53, 8421–8431. [PubMed]. doi:10.1021/jf050964f
- Krinsky, N., Landrum, J., & Bone, R. (2003). Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annual Review of Nutrition, 23, 171–201. doi:10.1146/annurev.nutr.23.011702.073307
- Kumaresan, A., Bujarbaruah, K.M., Pathak, K.A., Chhetri, B., Ahmed, S.K., & Haunshi, S. (2008). Analysis of a village chicken production system and performance of improved dual purpose chickens under a subtropical hill agro-ecosystem in India. Tropical Animal Health and Production, 40, 395–402. doi:10.1007/s11250-007-9097-y
- Lee, J., Koo, N., & Min, D.B. (2004). Reactive oxygen species, aging, and antioxidative nutraceuticals. Comprehensive Reviews in Food Science and Food Safety (CRFSFS), 3, 21–33. doi:10.1111/j.1541-4337.2004.tb00058.x
- Leeson, S. (2011). Nutrition and health: Poultry. Feedstuffs, 83, 52–60.
- Leeson, S., & Caston, L. (2004). Enrichment of eggs with lutein. Poultry Science, 83, 1709–1712. [PubMed]. doi:10.1093/ps/83.10.1709
- Leoncini, E., Edefonti, V., Hashibe, M., Parpinel, M., Cadoni, G., Ferraroni, M., … Boccia, S. (2015). Carotenoid intake and head and neck cancer: A pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. European Journal of Epidemiology, 31, 369–383. doi:10.1007/s10654-015-0036-3
- Li, B., Ahmed, F., & Bernstein, P.S. (2010). Studies on the singlet oxygen scavenging mechanism of human macular pigment. Archives of Biochemistry and Biophysics, 504, 56–60. doi:10.1016/j.abb.2010.07.024
- Li-Chan, E.C.Y., & Kim, H.O. (2008). Structure and chemical composition of eggs. In Y. Mine (Ed.), Egg bioscience and biotechnology (pp. 1–95). Hoboken, NJ: John Wiley & Sons.
- Lu, M.-S., Fang, Y.-J., Chen, Y.-M., Luo, W.-P., Pan, Z.-Z., Zhong, X., & Zhang, C.-X. (2015). Higher intake of carotenoid is associated with a lower risk of colorectal cancer in Chinese adults: A case-control study. European Journal of Nutrition, 54, 619–628. doi:10.1007/s00394-014-0743-7
- Ma, L., Dou, H.-L., Huang, Y.-M., Lu, X.-R., Xu, X.-R., Qian, F., … Lin, X.-M. (2012b). Improvement of retinal function in early age-related macular degeneration after lutein and zeaxanthin supplementation: A randomized, double-masked, placebo-controlled trial. American Journal of Ophthalmology, 154, 625–634. doi:10.1016/j.ajo.2012.04.014
- Ma, L., Dou, H.L., Wu, Y.Q., Huang, Y.M., Huang, Y.B., Zou, Z.Y., & Lin, X.M. (2012a). Lutein and zeaxanthin intake and the risk of age-related macular degeneration: A systematic review and meta-analysis. The British Journal of Nutrition, 107, 350–359. doi:10.1017/S0007114511004260
- Machmudah, S., & Goto, M. (2013). Methods for extraction and analysis of carotenoids. Natural Products, 3367–3411. doi:10.1007/978-3-642-22144-6_145
- Maggio, M., De Vita, F., Lauretani, F., Bandinelli, S., Semba, R.D., Bartali, B., … Ferrucci, L. (2015). Relationship between carotenoids, retinol, and estradiol levels in older women. Nutrients, 7, 6506–6519. doi:10.3390/nu7085296
- Mares, J. (2016). Lutein and zeaxanthin isomers in eye health and disease. Annual Review of Nutrition, 36, 571–602. doi:10.1146/annurev-nutr-071715-051110
- Mares-Perlman, J.A., Millen, A.E., Ficek, T.L., & Hankinson, S.E. (2002). The body of evidence to support a protective role for lutein and zeaxanthin in delaying chronic disease. Overview. The Journal of Nutrition, 132, 518S–524S. [PubMed].
- Meléndez-Martínez, A.J., Vicario, I.M., & Heredia, F.J. (2007). Review: Analysis of carotenoids in orange juice. Journal of Food Composition and Analysis, 20, 638–649. doi:10.1016/j.jfca.2007.04.006
- Meyers, K.J., Liu, Z., Millen, A.E., Iyengar, S.K., Blodi, B.A., Johnson, E., … Mares, J.A. (2015). Joint associations of diet, lifestyle, and genes with age-related macular degeneration. Ophthalmology, 122, 2286–2294. doi:10.1016/j.ophtha.2015.07.029
- Milani, A., Basirnejad, M., Shahbazi, S., & Bolhassani, A. (2016). Carotenoids: Biochemistry, pharmacology and treatment. British Journal of Pharmacology. 16 September 2016. [Epub ahead of print] . doi:10.1111/bph.13625
- Miranda, J.M., Anton, X., Redondo-Valbuena, C., Roca-Saavedra, P., Rodriguez, J.A., Lamas, A., … Cepeda, A. (2015). Egg and egg-derived foods: Effects on human health and use as functional foods. Nutrients, 7, 706–729. doi:10.3390/nu7010706
- Mugnai, C., Sossidou, E.N., Dal Bosco, A., Ruggeri, S., Mattioli, S., & Castellini, C. (2014). The effects of husbandry system on the grass intake and egg nutritive characteristics of laying hens. Journal of the Science of Food and Agriculture, 94, 459–467. doi:10.1002/jsfa.6269
- Nagao, A. (2011). Absorption and metabolism of dietary carotenoids. Biofactors, 37, 83–87. doi:10.1002/biof.151
- Namitha, K.K., & Negi, P.S. (2010). Chemistry and biotechnology of carotenoids. Critical Reviews in Food Science and Nutrition, 50, 728–760. doi:10.1080/10408398.2010.499811
- Nataraj, J., Manivasagam, T., Justin Thenmozhi, A., & Essa, M.M. (2015). Lutein protects dopaminergic neurons against MPTP-induced apoptotic death and motor dysfunction by ameliorating mitochondrial disruption and oxidative stress. Nutritional Neuroscience, 19, 237–246. [PubMed]. doi:10.1179/1476830515Y.0000000010
- National Agricultural Statistics Service (NAAS). (2013). Poultry production and value, NASS, USDA. Retrieved from www.nass.usda.gov/Publications/Todays_Reports/reports/plva0414
- Neacşu, A., Oprean, C., Curea, M., Tuchilă, G., & Trifu, M. (2003). Neuroprotection with carotenoids in glaucoma. Oftalmologia, 59, 70–75. [PubMed].
- Niclis, C., Díaz Mdel, P., Eynard, A.R., Román, M.D., & La Vecchia, C. (2012). Dietary habits and prostate cancer prevention: A review of observational studies by focusing on South America. Nutrition and Cancer, 64, 23–33. doi:10.1080/01635581.2012.630163
- Nimalaratne, C., Lopes-Lutz, D., Schieber, A., & Wu, J. (2011). Free aromatic amino acids in egg yolk show antioxidant properties. Food Chemistry, 129, 155–161. doi:10.1016/j.foodchem.2011.04.058
- Nimalaratne, C., Lopes-Lutz, D., Schieber, A., & Wu, J. (2012). Effect of domestic cooking methods on egg yolk xanthophylls. Journal of Agricultural and Food Chemistry, 60, 12547–12552. doi:10.1021/jf303828n
- Nimalaratne, C., Savard, P., Gauthier, S.F., Schieber, A., & Wu, J. (2015). Bioaccessibility and digestive stability of carotenoids in cooked eggs studied using a dynamic in vitro gastrointestinal model. Journal of Agricultural and Food Chemistry., 63, 2956–2962. doi:10.1021/jf505615w
- Nimalaratne, C., & Wu, J. (2015). Hen egg as an antioxidant food commodity: A review. Nutrients, 7, 8274–8293. doi:10.3390/nu7105394
- Nimalaratne, C., Wu, J., & Schieber, A. (2013). Egg yolk carotenoids: Composition, analysis, and effects of processing on their stability. In P. Winterhalter & S.E. Ebeler (Eds.), Carotenoid cleavage products, chapter 18 (pp. 219–225). Oxford: ACS Publications, Oxford University Press.
- Nkondjock, A., & Ghadirian, P. (2004). Intake of specific carotenoids and essential fatty acids and breast cancer risk in Montreal, Canada. The American Journal of Clinical Nutrition, 79, 857–864. [PubMed].
- Nolan, J.M., Loskutova, E., Howard, A., Mulcahy, R., Moran, R., Stack, J., … Beatty, S. (2015). The impact of supplemental macular carotenoids in Alzheimer’s disease: A randomized clinical trial. Journal of Alzheimer’s Disease, 44, 1157–1169. doi:10.3233/JAD-142265
- Nolan, J.M., Loskutova, E., Howard, A.N., Moran, R., Mulcahy, R., Stack, J., … Beatty, S. (2014). Macular pigment, visual function, and macular disease among subjects with Alzheimer’s disease: An exploratory study. Journal of Alzheimer’s Disease, 42, 1191–1202. doi:10.3233/JAD-140507
- Nolan, J.M., Meagher, K.A., Howard, A.N., Moran, R., Thurnham, D., & Beatty, S. (2016). Lutein, zeaxanthin and meso-zeaxanthin content of eggs laid by hens supplemented with free and esterified xanthophylls. Journal of Nutritional Science, 5, e1, 8 January 2016. doi:10.1017/jns.2015.35
- Okuyama, Y., Ozasa, K., Oki, K., Nishino, H., Fujimoto, S., & Watanabe, Y. (2014). Inverse associations between serum concentrations of zeaxanthin and other carotenoids and colorectal neoplasm in Japanese. International Journal of Clinical Oncology, 19, 87–97. doi:10.1007/s10147-013-0520-2
- Olson, J.B., Ward, N.E., & Koutsos, E.A. (2008). Lycopene incorporation into egg yolk and effects on laying hen immune function. Poultry Science, 87, 2573–2580. doi:10.3382/ps.2008-00072
- Ow, S.Y., Salim, M., Noirel, J., Evans, C., & Wright, P.C. (2011). Minimising iTRAQ ratio compression through understanding LC-MS elution dependence and high-resolution HILIC fractionation. Proteomics, 11, 2341–2346. doi:10.1002/pmic.201000752
- Owen, C.G., Jarrar, Z., Wormald, R., Cook, D.G., Fletcher, A.E., & Rudnicka, A.R. (2012). The estimated prevalence and incidence of late stage age related macular degeneration in the UK. The British Journal of Ophthalmology, 96, 752–756. doi:10.1136/bjophthalmol-2011-301109
- Packer, L., Weber, S.U., & Rimbach, G. (2001). Molecular aspects of alpha-tocotrienol antioxidant action and cell signaling. The Journal of Nutrition, 131, 369S–73S. [PubMed].
- Panasenko, O.M., Sharov, V.S., Briviba, K., & Sies, H. (2000). Interaction of peroxynitrite with carotenoids in human low density lipoproteins. Archives of Biochemistry and Biophysics, 373, 302–305. doi:10.1006/abbi.1999.1424
- Paul, R., & Borah, A. (2015). The potential physiological crosstalk and interrelationship between two sovereign endogenous amines, melatonin and homocysteine. Life Sciences, 139, 97–107. doi:10.1016/j.lfs.2015.07.031
- Perry, A., Rasmussen, H., & Johnson, E.J. (2009). Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. Journal of Food Composition and Analysis, 22, 9–15. doi:10.1016/j.jfca.2008.07.006
- Pintea, A., Dulf, F.V., Bunea, A., Matea, C., & Andrei, S. (2012). Comparative analysis of lipophilic compounds in eggs of organically raised ISA Brown and Araucana hens. Chemical Papers, 66, 955–963. doi:10.2478/s11696-012-0219-2
- Renzi, L.M., Dengler, M.J., Puente, A., Miller, L.S., & Hammond, B.R., Jr. (2014). Relationships between macular pigment optical density and cognitive function in unimpaired and mildly cognitively impaired older adults. Neurobiology of Aging, 35, 1695–1699. doi:10.1016/j.neurobiolaging.2013.12.024
- Rivera, S.M., & Canela-Garayoa, R. (2012). Analytical tools for the analysis of carotenoids in diverse materials. Journal of Chromatography A, 1224, 1–10. doi:10.1016/j.chroma.2011.12.025
- Rodriguez-Amaya, D.B. (1999). Changes in carotenoids during processing and storage of foods. Archivos Latinoamericanos De Nutrición, 49, 38S–47S. [PubMed].
- Rodriguez-Amaya, D.B. (2002). Effects of processing and storage on food carotenoids. Sight Life Newsletter (Special Issue), 3, 25–35.
- Rong, Y., Chen, L., Zhu, T., Song, Y., Yu, M., Shan, Z., … Liu, L. (2013). Egg consumption and risk of coronary heart disease and stroke: Dose-response meta-analysis of prospective cohort studies. BMJ, 346, e8539-e8539. doi:10.1136/bmj.e8539
- Ruxton, C. (2010). Recommendations for the use of eggs in the diet. Nursing Standard, 24, 47–56. [PubMed]. doi:10.7748/ns.24.37.47.s53
- Schieber, A., & Carle, R. (2005). Occurrence of carotenoid cis-isomers in food: Technological, analytical, and nutritional implications. Trends in Food Science & Technology, 16, 416–422. doi:10.1016/j.tifs.2005.03.018
- Schlatterer, J., & Breithaupt, D.E. (2006). Xanthophylls in commercial egg yolks: Quantification and identification by HPLC and LC-(APCI) MS using a C30 phase. Journal of Agricultural and Food Chemistry, 54, 2267–2273. [PubMed]. doi:10.1021/jf053204d
- Seddon, J.M., Ajani, U.A., Sperduto, R.D., Hiller, R., Blair, N., Burton, T.C., … Miller, D.T. (1994). Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA, 272, 1413–1420. [PubMed]. doi:10.1001/jama.1994.03520180037032
- Sen, S., & Chakraborty, R. (2011). Oxidative stress: Diagnostics, prevention and therapy. American Chemical Society, 1083, 1–37. doi:10.1021/bk-2011-1083
- Seuss-Baum, I. (2007). Nutritional evaluation of egg compounds. In R. Huopalahti, R. López-Fandiño, M. Anton, & R. Schade (Eds.), Bioactive egg compounds (pp. 117–144). Berlin, Germany: Springer-Verlag.
- Sharma, G.S., Kumar, T., Dar, T.A., & Singh, L.R. (2015). Protein N-homocysteinylation: From cellular toxicity to neurodegeneration. Biochimics Et Biophysica Acta, 1850, 2239–2245. doi:10.1016/j.bbagen.2015.08.013
- Sherwin, C.M., Richards, G.J., & Nicol, C.J. (2010). Comparison of the welfare of layer hens in 4 housing systems in the UK. British Poultry Science, 51, 488–499. doi:10.1080/00071668.2010.502518
- Shin, J.Y., Xun, P., Nakamura, Y., & He, K. (2013). Egg consumption in relation to risk of cardiovascular disease and diabetes: A systematic review and meta-analysis. The American Journal of Clinical Nutrition, 98, 146–159. doi:10.3945/ajcn.112.051318
- Silvera, S.A., Jain, M., Howe, G.R., Miller, A.B., & Rohan, T.E. (2006). Carotenoid, vitamin A, vitamin C, and vitamin E intake and risk of ovarian cancer: A prospective cohort study. Cancer Epidemiology, Biomarkers & Prevention, 15, 395–397. [PubMed]. doi:10.1158/1055-9965.EPI-05-0835
- Simon, H.-U., Haj-Yehia, A., & Levi-Schaffer, F. (2000). Role of reactive oxygen species (ROS) in the apoptosis induction. Apoptosis, 5, 415–418. [PubMed]. doi:10.1023/A:1009616228304
- Sisti, J.S., Lindström, S., Kraft, P., Tamimi, R.M., Rosner, B.A., Wu, T., … Eliassen, A.H. (2015). Premenopausal plasma carotenoids, fluorescent oxidation products, and subsequent breast cancer risk in the nurses’ health studies. Breast Cancer Research and Treatment, 151, 415–425. doi:10.1007/s10549-015-3391-6
- Song, W.O., & Kerver, J.M. (2000). Nutritional contribution of eggs to American diets. Journal of the American College of Nutrition, 19, 556–562. [PubMed]. doi:10.1080/07315724.2000.10718980
- Strati, I.F., Sinanoglou, V.J., Kora, L., Miniadis-Meimaroglou, S., & Oreopoulou, V. (2012). Carotenoids from foods of plant, animal and marine origin: An efficient HPLC-DAD separation method. Foods, 1, 52–65. doi:10.3390/foods1010052
- Sumner, D.A., Gow, H., Hayes, D., Matthews, W., Norwood, B., Rosen-Molina, J.T., & Thurman, W. (2011). Economic and market issues on the sustainability of egg production in the United States: Analysis of alternative production systems. Poultry Science, 90, 241–250. doi:10.3382/ps.2010-00822
- Surai, P.F., MacPherson, A., Speake, B.K., & Sparks, N.H. (2000). Designer egg evaluation in a controlled trial. European Journal of Clinical Nutrition, 54, 298–305. [PubMed]. doi:10.1038/sj.ejcn.1600939
- Swartz, M.E. (2005). UPLC™: An introduction and review. Journal of Liquid Chromatography & Related Technologies, 28, 1253–1263. doi:10.1081/JLC-200053046
- Tanvetyanon, T., & Bepler, G. (2008). Beta-carotene in multivitamins and the possible risk of lung cancer among smokers versus former smokers: A meta-analysis and evaluation of national brands. Cancer, 113, 150–157. doi:10.1002/cncr.23527
- The International Agency for the Prevention of Blindness. (2014). Vision WHO-international agency for prevention of blindness 2020. Retrieved from http://www.iapb.org/vision-2020
- Thurnham, D.I. (2007). Macular zeaxanthins and lutein – a review of dietary sources and bioavailability and some relationships with macular pigment optical density and age-related macular disease. Nutrition Research Reviews, 20, 163–179. doi:10.1017/S0954422407842235
- Trevithick-Sutton, C.C., Foote, C.S., Collins, M., & Trevithick, J.R. (2006). The retinal carotenoids zeaxanthin and lutein scavenge superoxide and hydroxyl radicals: A chemiluminescence and ESR study. Molecular Vision, 12, 1127–1135. [PubMed].
- United States Environmental Protection Agency (US-EPA). (2012). Poultry production phases. Retrieved from http://www.epa.gov/agriculture/ag101/poultryphases
- van Het Hof, K.H., West, C.E., Weststrate, J.A., & Hautvast, J.G. (2000). Dietary factors that affect the bioavailability of carotenoids. The Journal of Nutrition, 130, 503–506. [PubMed].
- van Ruth, S.M., Alewijn, M., Rogers, K., Newton-Smith, E., Tena, N., Bollen, M., & Koot, A. (2011). Authentication of organic and conventional eggs by carotenoid profiling. Food Chemistry, 126, 1299–1305. doi:10.1016/j.foodchem.2010.11.081
- Vishwanathan, R., Gendron, C.M., Goodrow-Kotyla, E.F., Wilson, T.A., & Nicolosi, R.J. (2010). Increased consumption of dietary cholesterol, lutein, and zeaxanthin as egg yolks does not decrease serum concentrations and lipoprotein distribution of other carotenoids, retinol, and tocopherols. Nutrition Research, 30, 747–755. doi:10.1016/j.nutres.2010.10.007
- Vishwanathan, R., Goodrow-Kotyla, E.F., Wooten, B.R., Wilson, T.A., & Nicolosi, R.J. (2009). Consumption of 2 and 4 egg yolks/d for 5 wk increases macular pigment concentrations in older adults with low macular pigment taking cholesterol lowering statins. American Journal of Clinical Nutrition, 90, 1272–1279. doi:10.3945/ajcn.2009.28013
- Walker, L.A., Wang, T., Xin, H., & Dolde, D. (2012). Supplementation of laying-hen feed with palm tocos and algae astaxanthin for egg yolk nutrient enrichment. Journal of Agricultural and Food Chemistry, 60, 1989–1999. doi:10.1021/jf204763f
- Wang, X.D. (2004). Carotenoid oxidative/degradative products and their biological activities. In N.I. Krinsky, S.T. Maynen, & H. Sies (Eds.), Carotenoids in health and disease (pp. 313–335). New York, NY: Marcel Dekker.
- Wang, T., Cai, H., Sasazuki, S., Tsugane, S., Zheng, W., Cho, E.R., … Epplein, M. (2016). Fruit and vegetable consumption, Helicobacter pylori antibodies, and gastric cancer risk: A pooled analysis of prospective studies in China, Japan and Korea. International Journal of Cancer. 19 October 2016. [Epub ahead of print] . doi:10.1002/ijc.20477
- Wang, L., Li, B., Pan, M.-X., Mo, X.-F., Chen, Y.-M., & Zhang, C.-X. (2014). Specific carotenoid intake is inversely associated with the risk of breast cancer among Chinese women. British Journal of Nutrition, 111, 1686–1695. [PubMed]. doi:10.1017/S000711451300411X
- Wenzel, M., Seuss-Baum, I., & Schlich, E. (2010). Influence of pasteurization, spray- and freeze-drying, and storage on the carotenoid content in egg yolk. Journal of Agricultural. Food Chemistry, 58, 1726–1731. doi:10.1021/jf903488b
- Wenzel, M., Seuss-Baum, I., & Schlich, E. (2011). Influences of storage time and temperature on the xanthophyll content of freeze-dried egg yolk. Food Chemistry, 124, 1343–1348. doi:10.1016/j.foodchem.2010.07.085
- Widomska, J., & Subczynski, W.K. (2014). Why has nature chosen lutein and zeaxanthin to protect the retina? Journal of Clinical & Experimental Ophthalmology, 5, 326. [PubMed]. doi:10.4172/2155-9570
- Widomska, J., Zareba, M., & Subczynski, W.K. (2016). Can xanthophyll-membrane interactions explain their selective presence in the retina and brain? Foods, 5(1), pii: 7. [PubMed]. doi:10.3390/foods5010007
- Yan, B., Lu, M.-S., Wang, L., Mo, X.-F., Luo, W.-P., Du, Y.-F., & Zhang, C.-X. (2016). Specific serum carotenoids are inversely associated with breast cancer risk among Chinese women: A case-control study. British Journal of Nutrition, 115, 129–137. [PubMed]. doi:10.1017/S000711451500416X
- Yeum, K.-J., & Russell, R.M. (2002). Carotenoid bioavailability and bioconversion. Annual Review of Nutrition, 22, 483–504. doi:10.1146/annurev.nutr.22.010402.102834
- Yuan, J.M., Stam, D.O., Arakawa, K., Lee, H.P., & Yu, M.C. (2003). Dietary cryptoxanthin and reduced risk of lung cancer: The Singapore Chinese Health Study. Cancer Epidemiology, Biomarkers & Prevention, 12, 890–898. [PubMed].
- Yue, X., Xu, Z., Prinyawiwatkul, W., & King, J.M. (2006). Improving extraction of lutein from egg yolk using an ultrasound-assisted solvent method. Journal of Food Science, 71, C239–C241. doi:10.1111/j.1750-3841.2006.00027.x
- Yuhas, R., McCormick, M., Yachetti, S., Burgher, A.M., Kong, K., & Walsh, J. (2011). A method for the measurement of lutein in infant formula. Food and Nutrition. Sciences, 2, 145–149. doi:10.4236/fns.2011.22020
- Zhang, S., Hunter, D.J., Forman, M.R., Rosner, B.A., Speizer, F.E., Colditz, G.A., … Willett, W.C. (1999). Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. JNCI Journal of the National Cancer Institute, 91, 547–556. [PubMed]. doi:10.1093/jnci/91.6.547
