 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Pine nut (Pinus pinea L.) is a nutritious, expensive tree nut. During storage, it is exposed to a wide range of environmental deteriorative conditions. This study describes the oxidative stability of pine nuts kept in pouches made of three different packaging materials and stored at three different temperatures. The packaging materials evaluated were low-density polyethylene (LDP), high-density polyethylene (HDP), and high-barrier metallized film (MF). Temperatures evaluated were 4, 20, and 60°C. In addition, a control unpacked sample was also evaluated in identical conditions. The oxidative parameters evaluated were acid value and peroxide value. Data analyses include a shelf-life study and a principal component analysis. The results obtained indicate what the best packaging material at 20°C was MF with a shelf-life of 703 d. At 4°C, the shelf-life of pine nuts stored in HDP was 1148 d. It is concluded that it is fundamental to use an adequate packaging to protect the seeds from environmental conditions that promote deterioration.
RESUMEN
El piñón de pino (Pinus pinea L.) es un fruto seco nutritivo, de alto precio. Durante su almacenamiento se exponen a condiciones ambientales que los deterioran. Este estudio describe la estabilidad oxidativa de piñones almacenados en envases elaborados con tres materiales, almacenados a tres temperaturas. Los materiales evaluados fueron polietileno de baja densidad (LDP), polietileno de alta densidad (HDP) y film metálico de alta barrera (MF). Las temperaturas evaluadas fueron 4, 20 y 60°C. Además, se evaluó una muestra control sin envase bajo idénticas condiciones. Los parámetros de oxidación evaluados fueron Índice de Acidez e Índice de Peróxidos. El análisis de los datos incluyó el estudio de vida útil y Análisis de Componentes Principales. Los resultados obtenidos indican que el mejor material de envasado a 20°C fue MF, con una vida útil de 703 d. A 4°C, la vida útil de los piñones almacenados en PAD fue 1148 d. Se concluye que es fundamental utilizar un envase adecuado para proteger el piñón de pino de las condiciones ambientales que promueven la oxidación.
PALABRAS CLAVES:
Introduction
Stone pine (Pinus pinea L.) is one of the nine major tree nut species worldwide and shows a high economic relevance. It is grown mainly in southern Europe, especially in Mediterranean countries such as Spain, Portugal, Italy, Greece, Albania, and Turkey (Evaristo, Batista, Correia, Correia, & Costa, Citation2010; Nunes, Quilhó, & Pereira, Citation1999; Özcan, Dağdelen, Kara, & Kanbur, Citation2013). Besides their outstanding organoleptic properties, nuts exhibit a high nutritional value, and there is growing evidence of the association of tree nuts consumption to a wide range of health benefits (Estruch et al., Citation2013; Jenkins et al., Citation2011; Rees et al., Citation2014; Sabaté & Ang, Citation2009). Moreover, although tree nuts are energy dense foods, their intake has been associated with reduced body mass index and advocated in weight maintaining diets (Lutz & Luna, Citation2016).
Most nuts have a long shelf-life if they are kept under optimum conditions. However, under unsuitable storage conditions, they soon become inedible, mainly due to staleness or rancidity, which is related to their high fat content. Pine nuts contain nearly 500 g fat kg−1, including unsaturated fatty acids, phytosterols, various tocopherols (α-, γ-, and δ-tocopherols), and squalene (Evaristo et al., Citation2010; Lutz, Álvarez, & Loewe, Citation2017; Maguire, O’Sullivan, Galvin, O’Connor, & O’Brien, Citation2004; Matthaus & Özcan, Citation2013; Nasri et al., Citation2009). Some of these molecules are good antioxidants, as is the case of tocopherols. In general terms, tocopherols are fairly unstable and may be degraded during storage, though their stability may increase at high temperatures if no oxygen is present (Sabliov et al., Citation2009).
The stability of tree nuts is affected by their fatty acid composition. Pine nuts possess a fatty acid profile in which polyunsaturated species are abundant, especially linoleic acid (18:2n − 6), with lesser amounts of α-linolenic acid (18:3n − 3). The major monounsaturated fatty acid is oleic acid (Evaristo et al., Citation2010; Kadri et al., Citation2015; Lutz et al., Citation2017; Nasri, Khaldi, Hammami, & Triki, Citation2005; Nergiz & Dönmez, Citation2004; Özcan et al., Citation2013; Ros, Citation2010; Ros & Mataix, Citation2006; Ryan, Galvin, O’Connor, Maguire, & O’Brien, Citation2006). The high levels of unsaturated fats limit the shelf-life of these nuts, making them prone to oxidation, which reduces their nutritional and organoleptic characteristics. During the storage and distribution steps, pine nuts are exposed to a wide range of environmental conditions that can trigger various chemical reactions that alter the product mainly due to oxidative processes (Jaya & Das, Citation2005).
Pine nut is a very expensive tree nut, and the global market demand for it is increasing worldwide (Lutz et al., Citation2017). It is important to preserve the quality of the seeds by storing them under adequate conditions in order to avoid lipid oxidation, since their fat content is high. Consequently, the packaging material used to preserve the quality of pine nuts is an effective way to establish a barrier to deteriorative conditions that promote lipid oxidation.
Taking into consideration the nutritional quality and health benefits of pine nuts, the aim of this study was to determine adequate storage conditions (temperature and packaging material) to preserve the lipid stability of pine nuts harvested in Chile, in order to extend their shelf-life.
Materials and methods
Materials
Pine nut (Pinus pinea L.) seeds were collected from June to November 2013, in three selected Chilean geographical growth macrozones defined as North (N): 31°–33° latitude; dry coast (DC): 34°–35° latitude; and South (S): 36°–38° latitude (Loewe, Balzarini, Alvarez, Delard, & Navarro-Cerrillo, Citation2016). Samples were gathered from Pinus pinea L. plantations, windbreaks, groves, and isolated trees. Cones were collected and pine nuts in shell kept in plastic nets at room temperature until they were manually shelled. Shelled pine nuts collected from the different macrozones were pooled.
Packaging materials
The packaging materials analyzed were low-density polyethylene (LDP), high-density polyethylene (HDP), and high-barrier metallized film (MF), all of which were commercially available. LDP and HDP were provided by Angel Plastics® (Valparaíso, Chile) and MF was provided by Alusa Techpack® (Santiago, Chile). The characteristics of these materials are shown in .
Table 1. Characteristics of the packaging materials studied.*
Tabla 1. Características de los materiales de envase estudiados.
The moisture of all the pine nut samples was evaluated AOAC 935.36b (Association of Official Analytical Chemists (AOAC), Citation2012). At the beginning of the study, pine nuts were standardized to 4% humidity. Then, 10 g of pine nuts was placed in a 10 cm × 10 cm pouch of each packing material with an average headspace of 4.5 cm3. The pouches were closed manually with a plastic sealing machine. Pine nuts sealed bags were held in controlled temperatures at 4, 20, or 60°C and analyzed after 7, 15, 30, 60, 90, and 120 d. A control sample of pine nuts was kept unpacked on a Petri dish under identical conditions.
Physical and chemical analysis
All reagents and solvents were analytical grade (Merck, Darmstadt, Germany) or Sigma Chemical Co (St. Louis, MO, USA).
Oil extraction
Seed oil was extracted according to Bligh and Dyer (Citation1959). Briefly, 8 g nuts was mixed in a solution containing 16 mL deionized water, 20 mL chloroform, and 40 mL methanol. The solution was stirred for 2 min in a homogenizer (MRC®, model HOG-020) at medium speed (15,000 rpm). Then, 20 mL chloroform was added, and the solution was stirred for 30 s in a homogenizer (MRC®, model HOG-020) at medium speed (15,000 rpm). After this, 20 mL deionized water was added, and the solution was stirred for 30 s in a homogenizer (MRC®, model HOG-020) at medium speed (15,000 rpm). The content was distributed in 50 mL tubes, and centrifuged at 4000 rpm for 10 min (Thermo Scientific, model Heraeus Megafuge 16R, Osterode, Germany). The chloroform layer was recovered, the lipid extracts were collected and chloroform was evaporated in a water bath with reciprocal agitation (Memmert, Hannover, Germany) at 60°C to get the dry extract.
Peroxide value
Peroxide value was assayed according to AOAC Method 965.33 (Association of Official Analytical Chemists (AOAC), Citation2012). Briefly, 2.0 ± 0.05 g oil was dissolved in 15 mL chloroform:acetic acid (3:2, v/v), 0.1 mL freshly prepared saturated KI solution was added, and the mixture was stirred occasionally. Then, 15 mL distilled water and the iodine was titrated with sodium thiosulfate (0.1 M). Peroxide value (PV) was calculated as
where M = mL Na2S2O3 for samples, B = mL Na2S2O3 for blank, N = molarity Na2S2O3 solution, m = sample weight (g).
Acid value
Acid value was assessed according to AOAC Method 940.28 (Association of Official Analytical Chemists (AOAC), Citation2012). For this purpose, 1–2 g oil was dissolved in 60 mL of alcohol and neutralized ether and the mixture was titrated with 0.25 M sodium hydroxide solution. Acid value (AV) was expressed as oleic acid, calculated as
where V NaOH = mL NaOH, M NaOH = NaOH molarity, f NaOH = NaOH factor, m = sample weight (g).
Statistical analysis
Stability study
Kinetic modeling for the degradation attributes was evaluated using regression analysis, including ANOVA (p < 0.05), F-ratios, coefficient of determination (R2), and residual analysis.
Complementing the above, the effect of packing material, temperature, and storage time on lipid oxidation parameters was evaluated using multifactorial ANOVA. The effects were evaluated through Fisher LSD intervals.
The degradation kinetics for the quality attributes were studied according to methodologies proposed by Robertson (Citation2010) and Labuza’s concept-based method (Labuza Citation1982; Labuza & Roboh Citation1982). The kinetic degradation of a food quality attribute (A) can be expressed as follows:
where A = quality attribute of interest, t = time, k = temperature-dependent constant, n = reaction order.
Kinetic orders were determined by the adjustment of the experimental data, solving the general expression of Equation (1) through a least square fitting procedure. Once the kinetic parameters were obtained, shelf-life was calculated for each of the attributes and storage conditions. Dependence of the temperature with the degradation kinetic was obtained with the Arrhenius model.
Multivariate data analysis
A dataset matrix was centered and scaled to unit variance. Following the kinetic modeling, a Principal component analysis (PCA) was performed as an exploratory tool to visualize the main variations among the samples, likely clusters, and particularly to reveal the weightiness relationship between variables. PCA analyses were validated by full cross-validation routines. Computations and adjustments were performed with Statgraphics Centurion XVII® (Statpoint Inc., 2010) Excel 2003® (Microsoft, 2009) and SIMCA-P + 12® (Umetrics AB, Sweden, 2008).
Results and discussion
Lipids oxidation
Oxidative stability of oils may be defined as their resistance to oxidation. It is an important indicator of shelf-life and depends on the composition of the sample and the conditions to which it is subjected (Guillén & Cabo, Citation2002). Lipid oxidation involves thermal degradation, hydrolytic reactions, and oxidative rancidity, generating a variety of by-products which increase during storage. In addition, this reaction causes deterioration in taste, flavor, odor, color, texture, and appearance, and a decrease in the nutritional value of the foods (Frankel, Citation1988). In this study, rancidity of pine nuts was evaluated by measuring AV and PV. Both values are useful measurable indices of food quality, and indicators of the initial stage of fat deterioration (Gotoh & Wada, Citation2006). AV reflects the degree of hydrolysis of fats resulting from enzyme reactions, catalyzed by heat and moisture, to produce free fatty acids. These products undergo further autoxidation to generate highly reactive molecules, responsible for producing unpleasant odors and flavors that affect the food quality (Brain & Yada, Citation2009; Gotoh & Wada, Citation2006). On the other hand, PV reflects primary lipid oxidation products generated early in the oxidative deteriorative process, during the initiation and propagation steps. PV is an index to quantify the amount of hydroperoxides generated in fats. The formation of hydroperoxides, primary oxidized products of lipids, must be suppressed to protect them against the formation of secondary oxidized products such as aldehydes, ketones, lactones, alcohols, acids, among others that affect the food quality and safety (Gotoh & Wada, Citation2006; Guillén & Cabo, Citation2002). Taking into consideration that moisture affects rancidity, the humidity of all pine nuts samples was standardized (4%).
shows the effects of temperature, time, and packaging material on the AV of pine nuts stored for 120 d. As expected, the initial value (t = 0) was very low (0.05 g OA/100 g oil). Free fatty acids increased in all pine nuts after 7 d, independently of the packaging material and temperature assayed, reaching an average of 0.55 g OA/100 g oil. After 60 d, the effect of temperature was clear: AV increased 28-fold in the nuts stored at 60°C, while the increase was lower at 4 and 20°C (9–16-fold). AV continued augmenting with time and at 120 d, the increase reached 76-fold. The effect of packaging material was also evident, and free fatty acids accumulated more in pine nuts stored in LDP, compared with HDP or MF. In these two pouches, AV increased 12, 29, and 32-fold at 4, 20, and 60°C, respectively. All pine nuts exhibited the highest AV when kept at 60°C. The effect of high temperature may be associated with the rise in water vapor within the pouch and oxygen permeability, both factors which accelerate the hydrolytic reactions (García, Martino, & Zaritzk, Citation2000; Sloan et al., Citation2016) and the generation of by-products associated with rancid taste and harmful effects on biological systems (Frankel, Citation1987; Kirk & Sawyer, Citation1991).
Figure 1. Acid value variation of pine nuts stored in different packing materials (a) and unpacked control (b) at various temperatures.
Figura 1. Variación del Índice de Acidez en piñones de pino almacenados en diferentes materiales (a) y en control sin empaque (b) a distintas temperaturas.
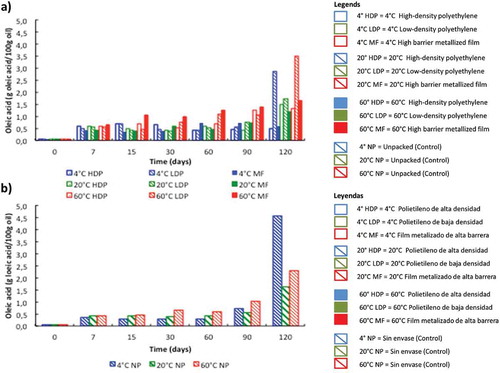
Similarly with our results, Gamli and Hayoğlu (Citation2007) reported that the rise of storage temperature led to an increase of free fatty acids in pistachio nut paste stored at 4 and 20°C within sealed glass jars, as well as in vacuumed and non-vacuumed polypropylene bags, observing that the rise of AV was lower in nut paste stored at 4°C compared with samples kept at 20°C. Additionally, Lin et al. (Citation2012) observed in almonds that high temperature and humidity favored biochemical reactions leading to free fatty acids formation. In our study, at the end of the storage test period, the unpacked pine nuts exhibited an increase of AV of 99, 35, and 50-fold at 4, 20, and 60°C, respectively.
Peroxides are major products of lipid oxidation, and the amount formed is a classic indicator of oxidative rancidity, although there is a relatively short induction period during which they form, accumulate, and dissipate (O’Brien, Citation1998). shows the effects of temperature, time, and packing material on PV of pine nuts stored during 120 d. The initial PV of pine nuts at t = 0 was 1.73 mEq peroxides kg−1 oil, increasing since day 7 between 1.1 and 3.4-fold in all the conditions assayed.
Figure 2. Peroxide value variation of pine nuts stored in different packing materials (a) and unpacked control (b) at various temperatures.
Figura 2. Variación del Índice de Peróxidos en piñones de pino almacenados en diferentes materiales (a) y en control sin empaque (b) a distintas temperaturas.
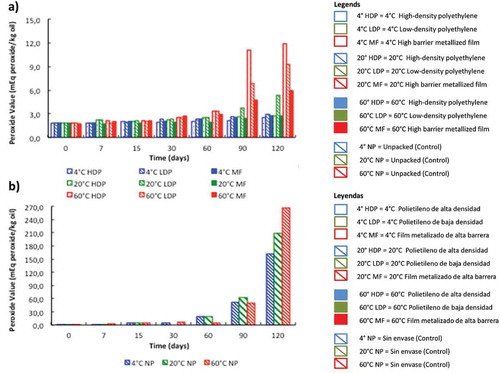
During the first 30 d of storage, no effects of the packaging material or temperature were observed on PV (). After 60 d, control pine nuts presented a significant increase in PV in comparison with the packed samples submitted to different temperatures (p < 0.05). The shelf-life limiting value of PV has not been clearly established. For instance, the limit PV in chips has been set at 25 mEq peroxide kg−1 oil (Ikpeme, Eneji, & Essiet, Citation2007), while in Brazil nuts, a group of trained panelists identified oxidized odors when the PV was 9.9 mEq kg−1 oil; however, the consumers only rejected the samples at 17.4 mEq kg−1 oil (Zajdenwerg, Branco, Alamed, Decker, & Castro, Citation2011). In the study of Gamli and Hayoğlu (Citation2007), a high storage temperature resulted in higher PV in pistachio nut paste. In our study, a significant rise of PV was observed at 60°C after 90 d (p < 0.05), while at lower temperatures, the PV variation was similar. After 90 d at 60°C, pine nuts stored in HDP showed the highest increase of PV, followed by LDP and MF, which induced the lowest rise (p < 0.05). After 120 d at 4 and 20°C, the protective effect of HDP and MF was similar (p > 0.05). These data indicate that both HDP and MF are adequate packaging materials to keep the quality of pine nuts stored at room temperature. However, if during the transport and/or distribution of the seeds they are exposed to higher temperatures, only MF pouches could contribute to minimize the lipid oxidative process, thus increasing the shelf-life of pine nuts.
After 120 d, PV values were higher in the nuts stored at 60°C (p < 0.05). At this high temperature, the pouches assayed were not effective due to the increased oxygen permeability of the packing under this condition, since the oxidative process is enhanced by the oxygen concentration surrounding the samples. Mexis, Badeka, Riganakos, Karakostas, and Kontominas (Citation2009) stored shelled walnuts at 4 and 20°C during 12 months, concluding that as the oxygen barrier of the packaging material decreased, the effect of temperature became more significant. Additionally, these authors emphasize that storage temperature exerted a more pronounced effect than light on food products packed in LDP. The ineffectiveness of the coatings at high temperature may be attributed in part to an augmented oxygen permeability of these materials with increased storage temperature.
After 120 d, PV increased significantly at the three temperatures assayed in the control nuts (p < 0.05). The PV values measured at 4, 20, and 60°C were 162.2, 208.7, and 267.0 (mEq peroxide kg−1 oil), respectively. These data represent an increase of 94, 121, and 154-fold in relation with the initial value (t = 0). The rise in storage temperature promotes the oxidation of fats, which produces alkyl peroxides and a variety of chemical species. The changes observed through time in both AV and PV in this study indicate that lipolytic activity augments with heating, and the rate of lipid oxidation nearly duplicates for every 10°C increase in temperature. PV values reveal that the oxidation level of pine nuts during storage could be lowered if the right packing is used, and storage temperature is adequate. It is necessary to take into consideration that a barrier to oxygen is required, since gas permeability of the packaging materials can be determinant of deteriorative reactions (Sacchetti, Cocci, Pinnavaia, Mastrocola, & Dalla Rosa, Citation2008). For instance, some edible coatings are effective in preventing oil oxidation in walnuts and pine nuts due to their oxygen barrier function, which is comparable to that of synthetic packaging materials (Mehyar, Al-Ismail, Han, & Chee, Citation2012).
Stability study
Kinetic modeling describes the reaction rate as a function of storage time and allows predicting changes in a particular food during storage. To predict these changes, the univariate adjusting kinetics was applied, using the methodology of Van Boekel (Citation2008). For this, 48 adjusts were realized for all the variables and conditions analyzed (data not shown). This implies that numerous kinetics assays had to be considered and, in some of them, adjusts were not very good; therefore, the capacity of these kinetic models to predict values of shelf-life could be calculated erroneously. This disadvantage was solved using a multivariate tool that allows obtaining a global perspective of the deterioration process: PCA, shown in .
Figure 3. Principal components analysis of parameters evaluated in pine nuts under different storage conditions.
Figura 3. Análisis de Componentes Principales de parámetros evaluados en piñones de pino bajo diferentes condiciones de almacenamiento.
(a) Correlation loading plot and scores biplot (correlation scaled) and (b) variable importance from X’s variables in latent projection.
a) Correlación entre contribución de cada variable y varianza (correlación escalada)b) Importancia de la variable desde las variables X’s en la proyección latente
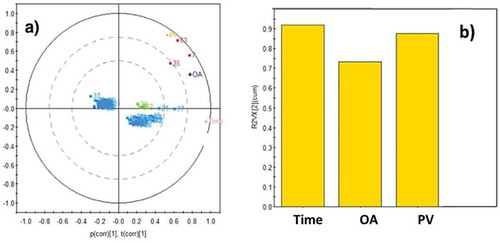
PCAs are linear combinations of the original variables and are determined so that the first principal components explain the largest part of the total variance. This means that correlated variables are explained by the same principal components and less correlated variables by different PC. shows the loading plot for the first two dimensions of the regular PCA model on all the quality variables. In the present study, the factorial model retained two principal components: Factor 1 (64.4% explained variance) arranges the samples according to time of deterioration, and Factor 2 (19.1% explained variance) shows a variable sorting according to temperature of deterioration and PV. The cumulative contribution rate of the two PC was 83.5% of the total variability, which appeared to provide enough information on the samples. The latent projection indicates that the value of the relation between the factors time and PV over the variable X was near 0.9. This analysis confirms the importance of PV (Factor 2) in relation with the deteriorative process, since lipid oxidation is a dynamic equilibrium process in which hydroperoxides are the key intermediates that control the progress of autoxidation.
indicates the kinetics parameters according to the type of packing and temperature of storage. These data and the shelf-life of pine nuts were calculated using PV, since PCA gave acceptable goodness of fit of these parameters in comparison with AV. In most cases, regression analysis showed a linear relationship between PV and time (high value of R2 of the model), adjusting these parameters appropriately into order zero kinetics (goodness of fit: p < 0.05), with the exception of the following samples: control at 4°C; control and LDP pouches at 20°C; and all the packages at 60°C. In all these cases, the kinetics observed was first order. Most quality-related reaction rates are either zero or first order, and the statistical difference between both may be small (Labuza & Roboh, Citation1982b).
Table 2. Kinetics parameters according to the type of packaging material and storage temperature.
Tabla 2. Parámetros cinéticos según el tipo de material de envase y temperatura de almacenamiento.
The data obtained strengthen the importance of the packaging material to maintain the shelf-life of pine nuts. Similarly, Senesi, Rizzolo, and Sarlo (Citation1991) reported that in peeled almond kept stored beyond 9 months, quality could be maintained only by using metallized pouches (low O2 permeability) and refrigeration. At the three temperatures assayed, the rate of deterioration associated to PV was higher in control nuts (p < 0.05). The oxidative process was evidenced through a shorter shelf-life, which did not exceed 37 d.
A higher value of k in control samples in comparison with all the packed nuts reveals a spontaneous deterioration process, which is consistent with the chemical and physical changes that took place in the non-protected seeds. At 4°C, the highest deterioration was observed in pine nuts kept in LDP pouch (p < 0.05). Their shelf-life reached 583 d, in comparison with the samples packed in HDP and MF, which reached 1148 and 704 d, respectively. These results highlight the importance of the packaging material to preserve the quality of pine nuts. Packing represents an important tool to protect pine nuts against moisture, oxygen, and the loss of nutritional value and organoleptic characteristics (mainly flavor and odor), since during their storage period, they are exposed to a wide range of environmental conditions.
The lowest shelf-life was observed in the samples stored at 60°C. However, MF preserved the quality attributes of pine nuts, given that the shelf-life was significantly higher: 583 d, while in LDP, it reached 102 d, and in samples kept in HDP, it reached 85 d only (p < 0.05). This may be related with the high temperature, which causes faster deterioration with a loss of nutritional and healthy characteristics. The study demonstrates that, under these conditions, MF is an appropriate protective barrier for pine nuts. At 20°C, the best packaging material was MF (703 d), followed by HDP (607 d) and LDP (163 d) (p < 0.05). It is important to notice that the shelf-life of nuts kept in MF pouches was similar at 4 and 20°C, reaching 704 and 703 d, respectively.
Conclusions
Due to the chemical characteristics of pine nuts, the storage conditions are determinant of their quality and shelf-life. The main cause of deterioration is lipid oxidation, and the increase in oxidation markers such as AV and PV confirms that it is fundamental to use an adequate packing to protect the seeds against environmental conditions that promote deterioration, such as moisture and oxygen.
The PCA model showed 2 principal components: Factor 1 (64.4% explained variance), arranging the samples according to time of deterioration, and Factor 2 (19.1% explained variance), showing a variable sorting according to temperature of deterioration and PV. The relevance of PV as an indicator of the presence of key processes that control the progress of autoxidation was confirmed.
During the storage period of pine nuts kept at 20°C, MF was the best material to preserve their quality of this valuable food, compared with LDP and HDP. However, at 4°C samples kept in HDP presented a higher shelf-life (1148 d) in comparison with the other materials assayed.
Acknowledgements
This work was supported by the Comisión Nacional de Investigación Científica y Tecnológica, Chile: [Grant Numbers FONDEF D11I1134] and CIDAF [CID 04/06].
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Association of Official Analytical Chemists (AOAC). (2012). Official methods of analysis of AOAC International (19th ed.; G. W. Latimer, Ed.). Gaithersburg, MD: AOAC International. ISBN: 0-935584-83-8.
- Bligh, E. G., & Dyer, W. J. (1959). A rapid method for total lipid extraction and purification. Canadian Journal of Physiology and Pharmacology, 37, 911–917. doi:10.1139/o59-099
- Brain, C. B., & Yada, R. Y. (2009). Food biochemistry. In G. Campbell-Platt (Ed), Food science and technology (pp. 57–84). West Sussex, UK: Wiley-BlackWell.
- Estruch, R., Ros, E., Salas-Salvadó, J., Covas, M. I., Corella, D., Arós, F., & Martínez-González, M. A. (2013). Primary prevention of cardiovascular disease with a Mediterranean diet. The New England Journal of Medicine, 368, 1279–1290. doi:10.1056/NEJMoa1200303
- Evaristo, I., Batista, D., Correia, I., Correia, P., & Costa, R. (2010). Chemical profiling of Portuguese Pinus pinea L. nuts. Journal of the Science of Food and Agriculture, 90, 1041–1049. doi:10.1002/jsfa.3914
- Frankel, E. N. (1987). Secondary products of lipid oxidation. Chemistry and Physics of Lipids, 44, 73–85. doi:10.1016/0009-3084(87)90045-4
- Frankel, E. N. (1988). Foods. In Lipid oxidation (First., pp. 187–225). Dundee: The Oil Press.
- Gamli, F., & Hayoğlu, I. (2007). The effect of the different packaging and storage conditions on the quality of pistachio nut paste. Journal of Food Engineering, 78, 443–448. doi:10.1016/j.jfoodeng.2005.10.013
- García, M. A., Martino, M. N., & Zaritzk, N. E. (2000). Lipid addition to improve barrier properties of edible starch-based films and coatings. Journal of Food Science, 65, 941–947. doi:10.1111/j.1365-2621.2000.tb09397.x
- Gotoh, N., & Wada, S. (2006). The importance of peroxide value in assessing food quality and food safety. Journal of the American Oil Chemists’ Society, 83, 473–474. doi:10.1007/s11746-006-1229-4
- Guillén, M., & Cabo, N. (2002). Fourier transform infrared spectra data versus peroxide and anisidine values to determine oxidative stability of edible oils. Food Chemistry, 77, 503–510. doi:10.1016/S0308-8146(01)00371-5
- Ikpeme, C. A. E., Eneji, C. A., & Essiet, U. (2007). Storage stability and sensory evaluation of taro chips fried in palm oil, palmolein oil, groundnut oil, soybean oil and their blends. Pakistan Journal of Nutrition, 6, 570–575. doi:10.3923/pjn.2007.570.575
- Jaya, S., & Das, H. (2005). Accelerated storage, shelf life and colour of mango powder. Journal of Food Processing and Preservation, 29, 45–62. 00012.x. 10.1111/j.1745-4549.2005
- Jenkins, D. J. A., Kendall, C. W. C., Banach, M. S., Srichaikul, K., Vidgen, E., Mitchell, S., & Josse, R. G. (2011). Nuts as a replacement for carbohydrates in the diabetic diet. Diabetes Care, 34, 1706–1711. doi:10.2337/dc11-0338
- Kadri, N., Khettal, B., Aid, Y., Kherfellah, S., Sobhi, W., & Barragan-Montero, V. (2015). Some physicochemical characteristics of pinus (Pinus halepensis Mill., Pinus pinea L., Pinus pinaster and Pinus canariensis) seeds from North Algeria, their lipid profiles and volatile contents. Food Chemistry, 188, 184–192. doi:10.1016/j.foodchem.2015.04.138
- Kirk, R. S., & Sawyer, R. (1991). Pearson’s composition and analysis of foods (9th ed.). London: Longman Scientific and Technical.
- Labuza, T. P. (1982). Shelf life dating of foods. Westport, CT, USA: Food and Nutrition Press Inc.
- Labuza, T. P., & Roboh, D. (1982). Theory and application of Arrhenius kinetics to the prediction of nutrient losses in foods. Food Technology, 36, 55–74.
- Lin, X., Wu, J., Zhu, R., Chen, P., Huang, G., Li, Y., & Ruan, R. (2012). California almond shelf life: Lipid deterioration during storage. Journal of Food Science, 77, C583–C593. doi:10.1111/j.1750-3841.2012.02706.x
- Loewe, M. V., Balzarini, M., Alvarez, C. A., Delard, R. C., & Navarro-Cerrillo, R. M. (2016). Fruit productivity of Stone pine (Pinus pinea L.) along a climatic gradient in Chile. Agricultural and Forest Meteorology, 223, 203–216. doi:10.1016/j.agrformet.2016.04.01
- Lutz, M., Álvarez, K., & Loewe, V. (2017). Chemical composition of pine nut (Pinus pinea L.) grown in three geographical macrozones in Chile. CYTA – Journal of Food, 15(2), 284–290. doi:10.1080/19476337.2016.1250109
- Lutz, M., & Luna, L. (2016). Nuts and body weight: An overview. Journal of Nutrition and Health, 3(104). doi:10.15744/2393-9060.3.104
- Maguire, L. S., O’Sullivan, S. M., Galvin, K., O’Connor, T. P., & O’Brien, N. M. (2004). Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. International Journal of Food Sciences and Nutrition, 55, 171–178. doi:10.1080/09637480410001725175
- Matthaus, B., & Özcan, M. M. (2013). Fatty acid, tocopherol and sterol contents of forest pine seed oil. Asian Journal of Chemistry, 25(17), 9845–9847. doi:10.14233/ajchem.2013.15503
- Mehyar, G., Al-Ismail, K., Han, J. H., & Chee, G. W. (2012). Characterization of edible coatings consisting of pea starch, whey protein isolate, and carnauba wax and their effects on oil rancidity and sensory properties of walnuts and pine nuts. Journal of Food Science, 77(2), E52–E59. doi:10.1111/j.1750-3841.2011.02559.x
- Mexis, S. F., Badeka, A. V., Riganakos, K. A., Karakostas, K. X., & Kontominas, M. G. (2009). Effect of packaging and storage conditions on quality of shelled walnuts. Food Control, 20, 743–751. doi:10.1016/j.foodcont.2008.09.022
- Nasri, N., Khaldi, A., Hammami, M., & Triki, S. (2005). Fatty acid composition of two Tunisian pine seed oils. Biotechnology Progress, 21, 998−1001. 05.023. 10.1021/bp049568s
- Nasri, N., Tlili, N., Ben Ammar, K., Khaldi, A., Fady, B., & Triki, S. (2009). High tocopherol and triacylglycerol contents in Pinus pinea L. seeds. International Journal of Food Sciences and Nutrition, 60(S1), 161–169. doi:10.1080/09637480802577854
- Nergiz, C. I., & Dönmez, I. (2004). Chemical composition and nutritive value of Pinus pinea L. seeds. Food Chemistry, 86, 365–368. doi:10.1016/j.foodchem.2003.09.009
- Nunes, E., Quilhó, T., & Pereira, H. (1999). Anatomy and chemical composition of Pinus pinea L. bark. Annals of Forest Science, 56(6), 479–484. doi:10.1051/forest:19990604
- O’Brien, R. D. (1998). Fats and oils analysis. In R. D. O’Brien (Ed), Fats and oils formulating and processing for applications (pp. 181–249). Lancaster, PA: Technomic Publishing Co. Inc.
- Özcan, M. M., Dağdelen, A., Kara, H. H., & Kanbur, G. (2013). The effect of microwave and roasted processing on fatty acid composition of oils extracted from stone pine (Pinus pinea) nuts. Rivista Italiana delle Sostanze Grasse, XC, 265–269.
- Rees, K., Hartley, L., Flowers, N., Clarke, A., Hooper, L., Thorogood, M., & Stranges, S. (2014). ‘Mediterranean’ dietary pattern for the primary prevention of cardiovascular disease (Review). Cochrane Database of Systematic Reviews, 2013(8, Art. No CD009825. John Wiley & Sons). doi:10.1002/14651858.CD009825.pub2
- Robertson, G. (2010). Food packaging and shelf life: A practical guide. Boca Raton, USA: Taylor and Francis Group.
- Ros, E. (2010). Health benefits of nut consumption. Nutrients, 2, 652–682. doi:10.3390/nu2070652
- Ros, E., & Mataix, J. (2006). Fatty acid composition of nuts. Implications for cardiovascular health. British Journal of Nutrition, 96, S29–S35. doi:10.1017/BJN20061861
- Ryan, E., Galvin, K., O’Connor, T. P., Maguire, O. R., & O’Brien, N. M. (2006). Fatty acid profile, tocopherol, squalene and phytosterol content of Brazil, pecan, pine, pistachio and cashew nuts. International Journal of Food Sciences and Nutrition, 57, 219–228. doi:10.1080/09637480600768077
- Sabaté, J., & Ang, Y. (2009). Nuts and health outcomes: New epidemiologic evidence. American Journal of Clinical Nutrition, 89, 1643S–1648S. doi:10.3945/ajcn.2009.26736Q
- Sabliov, C. M., Fronczek, C., Astete, C. E., Khachaturyan, M., Khachatryan, L., & Leonardi, C. (2009). Effects of temperature and UV light on degradation of α-tocopherol in free and dissolved form. Journal of the American Oil Chemists’ Society, 86, 895–902. doi:10.1007/s11746-009-1411-6
- Sacchetti, G., Cocci, E., Pinnavaia, G., Mastrocola, D., & Dalla Rosa, M. (2008). Influence of processing and storage on the antioxidant activity of apple derivatives. International Journal of Food Sciences and Technology, 43, 797–804. 2007.01518.x. 10.1111/j.1365-2621
- Senesi, E., Rizzolo, A., & Sarlo, S. (1991). Effect of different packaging conditions on peeled almond stability. Italian Journal of Food Sciences, 3, 209–218.
- Sloan, A. R., Dunn, M. L., Jefferies, L. K., Pike, O. A., Nielsen Barrows, S. E., & Steele, F. M. (2016). Effect of water activity and packaging material on the quality of dehydrated Taro (Colocasia esculenta (L.) Schott) slices during accelerated storage. International Journal of Food Sciences. doi:10.1155/2016/9860139
- Van Boekel, M. (2008). Kinetic modeling of food quality: A critical review. Comprehensive Reviews in Food Sciences and Food Safety, 7, 14–158. 2007.00036.x. 10.1111/j.1541-4337
- Zajdenwerg, C., Branco, G., Alamed, J., Decker, E. A., & Castro, I. A. (2011). Correlation between sensory and chemical markers in the evaluation of Brazil nut oxidative shelf-life. European Food Research and Technology, 233, 109–116. doi:10.1007/s00217-011-1493-x
