ABSTRACT
Effects of pomegranate seed oils extracted with supercritical fluid or cold press methods (SFE-PSO and CP-PSO), unsaponifiable (N), and fermented juice polyphenols (W) fractions of pomegranate as well as olive oil and alpha-tocopherol, as pure or in different solvents were evaluated on preventing copper-induced LDL oxidation. The results showed addition of pure SFE-PSO, CP-PSO, N fractions, and olive oil had no significant effect on LDL oxidation. Also using various solvents did not help in the term of extending the oxidation lag phase. However, adding 0.5, 1, 1.5, and 3 µL of the W solution in EtOH could delay the lag phase 103.5, 145.9, 200, and 199.1 min, respectively. There was no synergistic effect between W and N on inhibiting the oxidation. It can be concluded that polyphenolic fraction of pomegranate juice can act as antiatherogenic supplementation and natural preservative for meat and fatty foods through direct inhibition of LDL oxidation.
RESUMEN
A efectos de prevenir la oxidación de LDL inducida por cobre, el presente estudio se propuso evaluar los efectos que los aceites de semilla de granada extraídos con fluido supercrítico o métodos de presión en frío (SFE-PSO y CP-PSO), y las fracciones polifenólicas de jugo de granada fermentado e insaponificable (N), así como el aceite de oliva y el alfa-tocoferol, tienen en estado puro o en distintos solventes. Los resultados mostraron que la adición de fracciones SFE-PSO, CP-PSO, N puras y aceite de oliva no produjo ningún efecto significativo en la oxidación de LDL. Por otra parte, el uso de varios solventes no contribuyó a extender la fase de latencia de la oxidación. Sin embargo, al agregar 0.5, 1, 1.5 y 3 µl de la solución W en EtOH se pudo retrasar la fase de latencia 103.5, 145.9, 200 y 199.1 minutos, respectivamente. No se produjo ningún efecto sinérgico entre W y N en la inhibición de oxidación. Por lo tanto, se concluye que la fracción polifenólica del jugo de granada puede actuar como suplemento antiaterogénico y conservador natural de carnes y alimentos grasos mediante la inhibición directa de la oxidación de LDL.
Introduction
The pomegranate (Punica granatum Linnaeus), member of Punicacea family, is a well-known fruit in traditional medicine (Schubert, Lansky, & Neeman, Citation1999) and its protective and therapeutic effects on various diseases including cardiovascular and ischemic diseases, diabetes, obesity, Alzheimer, cancer, infertility, and arthritis have been documented previously (Cáceres, Girón, Alvarado, & Torres, Citation1987; Lad & Frawley, Citation1986; Lansky & Newman, Citation2007; Saxena & Vikram, Citation2004; Schubert et al., Citation1999).
Preliminary studies clearly showed high-antioxidant capacity of the pomegranate-derived products and thus paved the way for subsequent studies on detecting effective compounds and their possible mechanism of action as therapeutic agents (see review Haber, Joy, & Largent, Citation2011). In this context, pharmacological and toxicological activities of pomegranate compartments including juice, peel and seed have been evaluated (reviewed by Rahimi, Arastoo, & Ostad, Citation2012). Moreover, studies demonstrated presence of antioxidants such as steroids, flavonoids, polyphenols, saponins, alkaloids, triterpenoids, and vitamin C in various extractions from whole fruit, juice, peel and seeds of pomegranate (Bhandary, Kumari, Bhat, Sharmila, & Bekal, Citation2012). Among different pomegranate-derived products, considerable attention has been paid to the juice and its potent antioxidant capacity (Gil, Tomás-Barberán, Hess-Pierce, Holcroft, & Kader, Citation2000; Viuda-Martos et al., Citation2011) and also it has been claimed that the antioxidant activity of pomegranate juice is better than apple, black cherry, blueberry, cranberry, grape, and orange juices as well as red wine and green tea (Guo et al., Citation2008; Mori-Okamoto, Otawara-Hamamoto, Yamato, & Yoshimura, Citation2004; Seeram et al., Citation2008).
Recently pomegranate seeds which were considered as waste materials, create a new center of attention due to cheap availability, substantial amounts of antioxidants, unsaturated fatty acids, essential minerals, proteins and phytochemical compositions (El‐Nemr, Ismail, & Ragab, Citation1990; Fadavi, Barzegar, & Azizi, Citation2006; Özgül-Yücel, Citation2005). It has been shown that pomegranate seed oil (PSO) has wide range of beneficial biological attributes such as immunomodulatory and anti-inflammatory, anti-cancerous, and antioxidant properties (Kim et al., Citation2002; Kohno et al., Citation2004; Lansky & Newman, Citation2007; Singh, Chidambara Murthy, & Jayaprakasha, Citation2002; Yamasaki et al., Citation2006). Furthermore, considering oxidative modification hypothesis and role of Ox-LDL in atherosclerosis (Pirillo, Norata, & Catapano, Citation2013), many studies have been conducted on using various pomegranate-derived products as natural antioxidants to improve oxidative stress and decrease atherosclerosis risk factors. In-vivo and in-vitro studies have demonstrated attenuating and protective influences of the pomegranate products on LDL oxidation and atherosclerosis (Aviram et al., Citation2000; Basu & Penugonda, Citation2009; Davidson et al., Citation2009; Fuhrman, Volkova, & Aviram, Citation2005, Citation2010; Ignarro, Byrns, Sumi, De Nigris, & Napoli, Citation2006; Sezer, Akçay, Ilanbey, Yıldırım, & Sözmen, Citation2007) through various mechanisms, including induction of paraoxonase-1 (Aviram et al., Citation2004) and peroxisome proliferators activated receptor (Huang et al., Citation2005) activities as well as endothelial nitric-oxide synthase availability (De Nigris et al., Citation2006), and reduction of LDL aggregation (Aviram et al., Citation2000) and cellular uptake of oxidized LDL (Fuhrman et al., Citation2005). However, there is little information about direct effects of PSO on preventing LDL oxidation.
On the other hand, lipid oxidation is one of the main causes of deterioration in food quality decay especially in meat and fatty foods. Such an oxidation can lead to unpleasant flavors, low stability, and short shelf-life of the foods (Orhan, Aydin, Çölkesen, Sener, & Isimer, Citation2003). Although various synthetic preservatives are available to cope with these issues but there is a growing interest to use natural additives especially with plant source in food industries (Nasiri, Hesari, Shekarforoush, & Kooshesh, Citation2016; Nasiri, Moosavi-Nasab, Shekarforoush, & Golmakani, Citation2015; Naveena, Sen, Vaithiyanathan, Muthukumar, & Babji, Citation2007) due to general consumer acceptability and also risk of allergic reactions and severe disorders in asthmatic patients for artificial compounds (DeWitt, Citation1987). Various natural additives extract from grains, oil seeds, spices, honey, fruits, and vegetables have been applied and many promising results have been obtained (Naveena et al., Citation2007). Previously pomegranate extracts have been also used as the bio-preservatives for various foods such as meat, fishes, and cheese (Mahajan, Bhat, & Kumar, Citation2015; Naveena, Sen, Vaithiyanathan, Babji, & Kondaiah, Citation2008; Topuz, Yerlikaya, Ucak, Gumus, & Büyükbenli, Citation2014; Ünalan, Dalgaard, & Korel, Citation2011; Vaithiyanathan, Naveena, Muthukumar, Girish, & Kondaiah, Citation2011). Since the antioxidant capacity of various pomegranate extracts especially in direct inhibition of lipid oxidation is different, finding the best extract in this case could pave the way for using as a natural preservative compound.
Recent interests in using supercritical fluid extraction (SFE) method instead of mechanical cold press extraction (CP) in PSO extraction (Abbasi, Rezaei, & Rashidi, Citation2008; Liu, Xu, Hao, & Gao, Citation2009) might possibly result in a high-antioxidant activity of the oil in this new method (Chan & Ismail, Citation2009; Liu, Fu, et al., Citation2009). In the present study, we evaluated antioxidant potency of SFE- and CP-extracted pomegranate seed oils (SFE-PSO and CP-PSO), CP-PSO unsaponifiable fraction (N) as well as pomegranate fermented juice polyphenols fraction (W) in this in-vitro study. We utilized alpha-tocopherols and olive oil as controls in preventing copper-catalyzed oxidation of LDL; moreover, the effects of using various solvents on antioxidant potency of the pomegranate extractions were also evaluated.
Experimental
Samples
The venous blood samples from healthy volunteers were taken after 12-h overnight fast and collected in tubes contained EDTA. The plasma separation was carried out by subsequent centrifugation (10 min, at 1500× g).
LDL fraction isolation, purification and determination of concentration
Immediately after the plasma collection, the LDL isolation was performed by density gradient ultracentrifugation according to the method described by Abbey et al (Abbey, Nestel, & Baghurst, Citation1993). The density of the plasma was adjusted to 1.21 g/mL by adding KBr and then the plasma was layered under NaCl solution (d = 1.006 g/mL) containing 0.1% EDTA in Quickseal tubes. The LDL separation was performed by sequential ultracentrifugation (Beckman Ultracentrifuge, type L5-75, Ti 75 rotor) at 4°C and 280,000 × g for 6 h. LDL appeared as a clearly visible yellow band and was collected with a needle and syringe. The gel filtration method was used to purify the LDL particles. After two times rinsing the gel bed columns with PBS buffer, 800 μL of LDL were pipetted into the column and then washed with 2.6 mL of PBS and eluted. The purity of the LDL was tested by electrophoretic separation in an albumin containing agar-agarose gel and it was shown that the LDL content in the eluates was always above 96%. The extracted LDL cholesterol concentration was determined by enzymatic colorimetric method using automatic analyzer (Olympus AU 2700, NY, USA).
Preparation of pomegranate extractions
In order to compare effect of PSO and the oil extraction method on antioxidant potency of the products we used PSOs extracted with supercritical fluid extraction (SFE) and cold press extraction (CP) methods (SFE-PSO and CP-PSO). Also we tested PSO unsaponifiable fraction (N) alone to exclude saponifiable fraction effect which is susceptible to oxidation and possibly could affect the study results. Antioxidant effects of the polyphenols fraction of pomegranate fermented juice (well-known as a strong antioxidant derived from the fruit), olive oil (control oil) and alpha-tocopherol (well-known as a lipid soluble antioxidant) were also evaluated. All extractions were provided by Rimonest Ltd (Haifa, Israel). Different amount of the extractions (0.25–12 µL) based on their ability in preventing LDL oxidation were tested. Also their efficiency was evaluated as solution in different solvents such as ethanol (EtOH), isooctane, chloroform, and tween 80.
Evaluation of LDL oxidation delay
Using Cu2+ causes peroxidation of LDL and production of dienes which have maximum absorbance at 234 nm. The absorbance changing during a time course could be categorized in three phases including lag, propagation and decomposition (Esterbauer, Gebicki, Puhl, & Jürgens, Citation1992). In the lag phase which is stage of resistance to oxidation, the absorbance is almost constant (increases very slowly), but in the propagation phase, the absorbance increases very fast and reaches a maximum level. This phase is followed by decomposition of lipid hydroperoxides and producing aldehydic products. In this study extension of the lag phase in the presence of abovementioned extractions was measured. For this purpose, first calculated volume of each eluate (containing 0.08 mg LDL-cholesterol) was added to O2 saturated PBS buffer and then various amounts of oils and fractions as pure or in a solvent were added (final volume of reaction was 1 mL). Oxidation was initiated by adding CuSO4 to the solution. Reaction batches were thoroughly mixed and pipetted into quartz cuvettes; absorbance at 234 nm was measured every 5 min for 7 h at 30°C. To avoid possible contamination and errors, at the same time, 8 reaction batches (cuvettes) including two cuvettes without the solutions, two with only solvent, and four with the corresponding test solutions were measured in parallel. Besides, all reactions were double-measured. As described in our previous study (Ji et al., Citation2017), the intercept of the baseline and slope of the absorbance curve in the propagation phase was considered as the lag phase. The peak time was defined as the time of maximum absorbance.
Statistical analysis
Kolmogorov-Smirnov test was used to confirm the normal distribution of data. One-Way ANOVA test following Tukey’s post-hoc test was applied to compare the data among groups. All Statistical analysis was conducted using SPSS (version 16). Differences between means were considered significant when p-value was 0.05 or less.
Results
Effect of CP-PSO on LDL oxidation
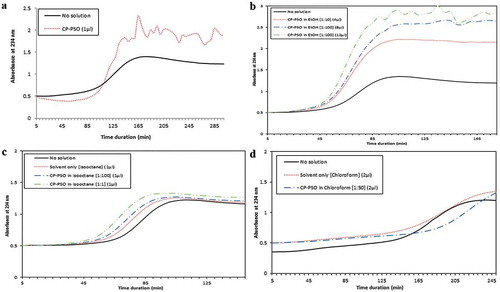
To evaluate a possible effect of CP-PSO on preventing copper-induced LDL oxidation we first added 1 µL of the CP-PSO oil directly (without solvent) to the reaction and the result showed no significant delay in oxidation (). Lack of a significant change in lag time led us to use the oil in combination with various solvents such as ethanol (EtOH), isooctane and chloroform. As shown in , EtOH alone had no effect on oxidation process of LDL and also dissolving the CP-PSO in this solvent with ratios of 1:10 or 1:100 did not cause a significant change in the lag phase duration. The same results were obtained for isooctane, as we found no influence of isooctane on LDL oxidation. Furthermore, using isooctane as solvent with ratios of 1:100 and 1:1 (CP-PSO/isooctane) did not have a beneficial effect on LDL oxidation (). The CP-PSO dissolved in chloroform (1:50) as well as chloroform alone also did not yield significant results ().
Effect of SFE-PSO on LDL oxidation
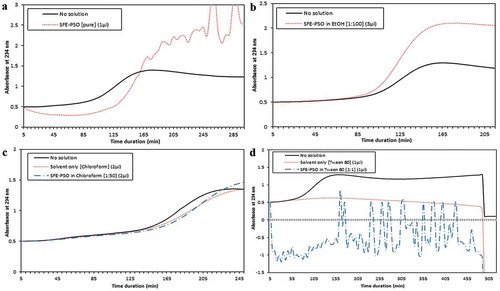
Adding SFE-PSO directly to the reaction showed no significant antioxidant properties and did not change the lag phase duration. However, it should be noted that because of fluctuation of the absorbance possibly due to lack of solvent, the assay could be poorly done (). In continue we dissolved the oil in EtOH (1:100) and chloroform (1:50); we did not find any significant antioxidant effect of SFE-PSO even as a solution (). Considering the lack of significant results, we also used tween 80 as a solvent (1:1); nevertheless, clear results could not be obtained due to high fluctuation of absorbance ().
Figure 2. Efecto del aceite de semillas de granada extraídas con fluido supercrítico (SFE-PSO) (a) puro o disuelto en (b) etanol, EtOH, (c) cloroformo y (d) tween 80 en la oxidación de LDL inducida por cobre. SFE-PSO (puro o en solventes) no ha tenido un efecto significativo en la duración de la fase de latencia de oxidación.
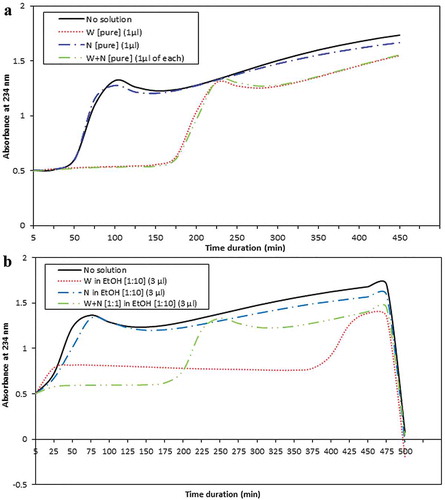
Effect of nonsaponifiable part of CP-PSO on LDL oxidation
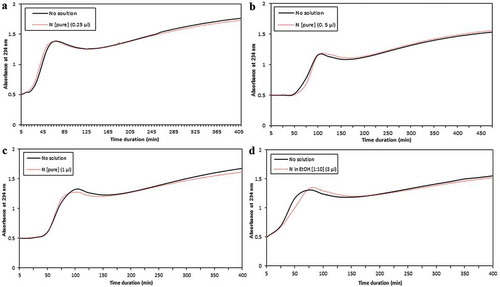
To find out if the nonsaponifiable part of CP-PSO (N) which does not contain fatty acids could directly prevent copper-induced oxidation of LDL we added 0.25, 0.5 or 1.5 µL of N with a concentration of 8 mg/mL to the reaction and obtained no significant result in the term of delaying LDL oxidation (). Furthermore, we added 3 µL of N/EtOH solution [1:10] to the reaction to determine if adding N in the form of dissolved in EtOH would show antioxidant properties; this time we also did not find any significant effect ().
Figure 3. Efecto de la fracción insaponificable del aceite de semillas de granada (N) tanto pura con cantidades de (a) 0,25, (b) 0,5 y (c) 1 μL como (d) disuelto en etanol(EtOH) en la oxidación de LDL inducida por cobre. La fracción N (pura o en solventes) no ha tenido un efecto significativo en la duración de la fase de latencia de oxidación.
Effect of polyphenols fraction of pomegranate fermented juice on LDL oxidation
Another pomegranate-derived product that we tested for possible ability in inhibition of LDL oxidation was polyphenols fraction of pomegranate fermented juice (W) which is well-known for its antioxidant capacity. After preparation of W solution in EtOH with a final concentration of 2 mg/mL, we added 0.5, 1, 1.5, or 3 µL of the solution to LDL oxidation reaction and interestingly observed that it could delay the lag phase respectively 103.5 ± 2.1, 145.9 ± 3.0, 200.0 ± 7.0, 198.1 ± 16.3, and 142.9 ± 16.7 min (). Our results showed that with increasing volume of the W fraction in the reaction, the lag phase duration also significantly increased (p < 0.05). However, the duration difference between reactions with 1.5 and 3 µL of W was not statistically different (p > 0.05, ). We found almost same trend of difference in the case of peak time as the reaction with 3 µL of W caused the highest peak time compared to the reactions with lower amounts (p < 0.05, ).
Table 1. Comparación de la duración de la fase de latencia y el pico de tiempo de la oxidación de los lipoproteínas de baja densidad del colesterol inducida por cobre entre las reacciones con diferentes extractos de granada y alfa-tocoferol.
Figure 4. Efecto de la fracción de polifenol les de zumo fermentado de granada (W) y disuelto en etanol (EtOH) con volúmenes de (a) 0.5, (b) 1, (c) 1.5 y (d) 3 μL en oxidación de LDL inducida por cobre. La adición de la fracción W (disuelta en EtOH [1:10]) con volúmenes de 0.5, 1, 1.5 y 3 μL ha extendido la fase de latencia de oxidación durante 103.5, 145.9, 200 y 198.1 min, respectivamente.
![Figure 4. Efecto de la fracción de polifenol les de zumo fermentado de granada (W) y disuelto en etanol (EtOH) con volúmenes de (a) 0.5, (b) 1, (c) 1.5 y (d) 3 μL en oxidación de LDL inducida por cobre. La adición de la fracción W (disuelta en EtOH [1:10]) con volúmenes de 0.5, 1, 1.5 y 3 μL ha extendido la fase de latencia de oxidación durante 103.5, 145.9, 200 y 198.1 min, respectivamente.](/cms/asset/7a4bca2d-38cf-4856-b01f-cd4eeb635a79/tcyt_a_1415375_f0004_c.jpg)
Synergistic effect of polyphenols fraction of pomegranate fermented juice and nonsaponifiable part of CP-PSO (W + N) on LDL oxidation
We evaluated a possible synergistic effect between W and N; when W and N were simultaneously added (1 µL of each), the lag phase duration was not different from that with 1 µL of W alone (). Furthermore, as shown in , we tested the potential synergistic effect when the fractions were added as solution in EtOH and interestingly the results demonstrated that the lag phase duration in reaction with W + N (1.5 µL of each) was significantly lower than the reaction with 1.5 µL W fraction only (p < 0.05, ). However, the peak time in the reaction with both 1.5 µL W and 1.5 µL N was comparable with the reaction contained only 1.5 µL W (p > 0.05, ).
Effects of olive oil and alpha-tocopherol on LDL oxidation
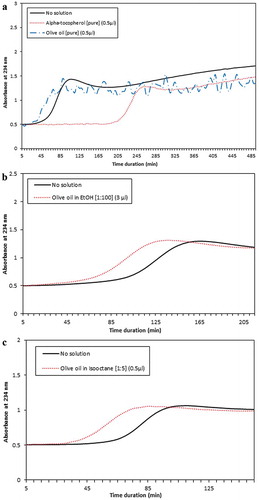
To demonstrate if olive oil as a control oil as well as alpha-tocopherol as a well-known lipid soluble antioxidant unlike PSO could affect LDL oxidation process or not, we included them in the assay. First we added 0.5 µL of alpha-tocopherol (1 mg/µL) or olive oil into the reaction and found that this amount of alpha-tocopherol could delay the lag phase for 267.5 min which is significantly higher than reactions with 0.5–3 µL of the W fraction (p < 0.05). However, the pick time was comparable with the reaction contained 3 µL of W (). About the olive oil we did not observe a significant effect; possibly because of absorbance fluctuation due to lack of solvent (). Considering the unevaluable result obtained for olive oil, the oil was dissolved in EtOH with a ratio of 1:100 and then 3 µL of the prepared solution were added to the reaction; again, no significant difference in the lag phase duration compared with the reaction without additive was observed (). We also changed the solvent to isooctane with a ratio of 1:5 and this time added 0.5 µL of the solution, but again no significant outcome was yielded ().
Figure 6. Efecto de (a) alfa-tocoferol puro y aceite de oliva, así como aceite de oliva disuelto en (b) etanol, EtOH o (c) isooctano en la oxidación de LDL inducida por cobre. El aceite de oliva puro o en solución no ha tenido un efecto significativo en la duración defase de latencia de oxidación La adición de alfa-tocoferol ha extendido la fase de latencia de oxidación de 267.5 min.
Discussion
There is still a great deal of interest in using pomegranate-derived products as protective and therapeutic compounds for different health complications as well as food bio-preservatives. Uncontrolled lipid peroxidation as an initial step of oxidative stress is involved in atherosclerotic diseases and many in-vivo studies have been developed to find out beneficial effects of pomegranate-derived products on oxidative status as well as their underlying mechanisms. Also lipid oxidation can be considered as the main issue in food preservation and in order to solve this problem pomegranate extracts have been also applied. To find out the best extract of pomegranate in inhibition of lipid oxidation we investigated effects of SFE-PSO, CP-PSO, N, and W fractions of pomegranate as pure or in various solvents, on preventing copper-induced LDL oxidation in vitro.
In the first part of our study we found that adding pure PSO extracted by either CP or SFE methods could not prevent LDL oxidation. These results were not in accordance with previously published data about ability of PSO in scavenging 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2'-azinobis-(3-ethylbenz-thiazoline-6-sulfonic) diammonium salt (ABTS) radicals (Dadashi, Mousazadeh, Emam-Djomeh, & Mousavi, Citation2013; Liu, Xu, Gong, He, & Gao, Citation2012). Considering that about 92% of antioxidant activity of pomegranate extracts is dependent on the polyphenolic compounds (Afaq, Saleem, Krueger, Reed, & Mukhtar, Citation2005; Lansky & Newman, Citation2007) it is necessary to use appropriate solvents in which the phenolic compounds are biologically active; therefore we repeated the tests with PSOs dissolved in EtOH as well as chloroform, based on previous in-vitro studies that had already used both substances as solvents (Dadashi et al., Citation2013; Liu et al., Citation2012). However, similar results were obtained when we used solvents such as EtOH, isooctane and chloroform. Another possible explanation for such an ineffective role of PSOs in preventing LDL oxidation in vitro could be potential oxidative sensitivities of the oils due to containing high amounts of unsaturated fatty acids such as punicic acid (Dadashi et al., Citation2013); thus, oxidation of the fatty acids during extraction, storage or even over the experiments is conceivable. In support of this hypothesis, Habibnia, Ghavami, Ansaripourc, and Vosough (Citation2012) have mentioned low stability of PSO in comparison with other edible oils and hence suggested necessity of keeping the oil in closed vessel as well as in cold, dry and dark places. In the present study, the unsaponifiable fraction of CP-PSO (N) was also tested in order to exclude possible deteriorating effects of unsaturated fatty acids. The unsaponifiable fraction constitutes less than 2% of the PSO (Habibnia et al., Citation2012) and contains phenols, sterols, tocopherols, hydrocarbons, triterpene, alcohols and squalene (Caligiani, Bonzanini, Palla, Cirlini, & Bruni, Citation2010). However, we did not observe any significant effect of pure N fraction in different concentrations as well as its solution in EtOH on delaying the lag phase of LDL oxidation; thus, the hypothesis about possible oxidation of unsaturated fatty acids was rejected. Also due to obtaining similar results for oils extracted by two different methods (CP or SFE), efficiency of the extraction methods could not be the reason. Besides, no significant variation in chemical profiles of PSOs extracted with the two methods has been documented previously (Albrecht et al., Citation2004). When we used olive oil as control oil, we again observed no significant effect on LDL oxidation which could confirm the obtained results about PSOs. As we expected alpha-tocopherol at low concentration could significantly prevent the LDL oxidation and delay the lag phase for about 267 min. Protective effect of alpha-tocopherol against copper-induced oxidation of LDL has been shown previously (Konishi, Hitomi, & Yoshioka, Citation2004). It has been revealed that alpha-tocopherol could have such an inhibitory effect through acting as a scavenger for the chain-carrying lipid peroxyl radicals and thus terminating the lipid peroxidation (Zhang, Chen, Yang, Chen, & Ying, Citation2014).
On the other hand, we found that the W fraction of pomegranate even at low amount (0.5 µL with a concentration of 0.2 mg/mL) could extend lag phase of LDL oxidation for 103.5 ± 2.1 min. Also we observed that the delay duration is dose dependent as with increasing volume of the W solution to 3 µL, the delay was extended to 198.1 ± 16.3 min. Antioxidant effects of pomegranate juice especially on lipids have been well documented, as Aviram et al. (Citation2000) reported that two-weeks consumption of the juice could significantly reduce oxidation of LDL and HDL in human. Furthermore, this group has shown an inhibitory effect of pomegranate juice consumption on macrophage-mediated LDL oxidation in mice (Aviram et al., Citation2000). Several studies have even claimed that antioxidant ability of the pomegranate juice is more potent than red wine, green tea as well as many fruit juices (Azadzoi, Schulman, Aviram, & Siroky, Citation2005; Gil et al., Citation2000; Rosenblat & Aviram, Citation2006). Also in well accordance with our findings, it has been indicated that pomegranate juice could inhibit cooper-induced oxidation of LDL by >90% (Aviram et al., Citation2008; Fuhrman & Aviram, Citation2001). Furthermore, Sezer et al.(Citation2007) have reported high ability of pomegranate fermented juice in decreasing LDL-diene levels. This group has suggested that such ability in reducing oxidized LDL attributed to the total phenol content. Owing that the W is polyphenolic fraction of pomegranate fermented juice, such an inhibitory effect even at low concentration seems logical. However, it should be mentioned that polyphenols are extensively metabolized in gastrointestinal tract and in most of the cases the chemical forms present in the juice are not the same in systemic circulation and possibly they do not have the same antioxidant capacity. So, further studies are required to clarify if the polyphenols of pomegranate in the blood circulation existing chemical forms can also have such a LDL oxidation inhibitory effect or not. However, there is possibility of absorption of some of polyphenolic compounds in gastrointestinal tract without any metallization. For instance, gallic and ellagic acids that have been mentioned as effective antioxidants in pomegranate juice (Aviram et al., Citation2008) can be directly absorbed by intestinal tract and increase the corresponding circulation levels (Konishi et al., Citation2004; Zhang et al., Citation2014). It has been reported that the maximum concentration (Cmax) of gallic acid in human plasma following oral administration of 50 mg galic acid was about 0.3 mg/L (Shahrzad & Bitsch, Citation1998). Considering that half maximal inhibitory concentration (IC50) of gallic acid for inhibition of LDL oxidation is 0.02 mg/L (Aviram et al., Citation2008), it could be postulated that consumption of the W fraction and its direct absorption can lead to increase in blood levels of gallic acid and consequently inhibit LDL oxidation. Almost same explanation could be considered for ellagic acid, although its Cmax in human plasma following administration of 25 mg ellagic acid was about 0.03 mg/L which is lower than gallic acid (Seeram, Lee, & Heber, Citation2004). However, due to high concentration of this acid in pomegranate juice (about 121 mg/L in commercial juice from concentrate) (Gil et al., Citation2000), consumption of the W fraction possibly can increase direct intestinal absorption and plasma Cmax and consequently prevent LDL oxidation.
In the term of bio-preservation, Naveena et al. (Citation2008) showed that even low levels of pomegranate juice have the sufficient capacity to inhibit oxidation of chicken patties. In good agreement with our findings, Vaithiyanathan et al. (Citation2011) demonstrated that the W fraction of pomegranate at the concentration of 0.02% could reduce the spent hen breast meat oxidation and prolong shelf life of the chicken meat.
Studies have attributed the inhibitory influences of pomegranate extractions against copper-induced LDL oxidation to existence of several compounds such as gallic, glucopyranose, punicalagin, punicalin, and ellagic acid (Aviram et al., Citation2008). Having no significant effect of PSOs, N and also olive oil on preventing LDL oxidation which was observed in this study could be because of lacking abovementioned components in these extractions. In support of this explanation, none of the abovementioned compounds have been detected in PSO (Rahimi et al., Citation2012). Although it should be mentioned that our results proved inability of PSOs in directly preventing copper-induced LDL oxidation in vitro and this does not mean that PSOs generally have no beneficial effect on LDL oxidation, especially in vivo. However, several studies have shown antioxidant effects of PSO through reducing cyclooxygenase and lipoxygenase enzyme activities (Schubert et al., Citation1999). Besides, we examined low concentration of the oils and it could be that the concentration was not sufficient to prevent LDL oxidation; so, further studies with higher concentration of PSO or its N fraction are required.
In vivo, different mechanisms have been suggested for antiatherogenic activity of pomegranate extractions, especially juice, such as reduction of oxidant enzyme activities (Schubert et al., Citation1999), inducing antioxidant enzymes expression (Khateeb, Gantman, Kreitenberg, Aviram, & Fuhrman, Citation2010), enhancing nitric oxide activity (Ignarro et al., Citation2006), inhibiting cellular uptake of oxidized LDL and cholesterol biosynthesis (Fuhrman et al., Citation2005). However, the present in-vitro study showed a direct inhibitory effect of the W fraction and not PSOs of pomegranate on copper-induced LDL oxidation. Such an antioxidant effect which most likely was a subsequent effect of polyphenolic compounds could be achieved by previously suggested mechanisms, including (1) chelation of copper ions by phenolic compounds and (2) scavenging of free radicals via hydrogen donation of phenolic hydroxyl groups (Fuhrman et al., Citation2010).
It has been demonstrated that pomegranate juice is more potent than purified phenolic compounds in the term of antioxidant and antiatherogenic properties (Aviram et al., Citation2008; Seeram et al., Citation2005). Besides, combined action among different substances in the pomegranate extractions has been suggested (Zahin, Aqil, & Ahmad, Citation2010). Owing to the above-mentioned evidences and in order to find out if phenolic compounds of seed and juice have possible cooperation together or not, we examined a potential synergistic effect of W and N fractions on delaying LDL oxidation. Although our results showed no positive synergistic effects in vitro, possible synergistic influences under in-vivo conditions are not excluded.
In conclusion, our results showed a potent antioxidant effect of pomegranate W fraction on cooper-induced LDL oxidation in vitro. The PSOs extracted with either CP or SFE methods as well as N fraction of the oils did not indicate such antioxidant influence. It could be concluded that polyphenolic fraction of pomegranate juice can act as an important biofood preservative by reducing oxidative process. Furthermore, human intervention studies support the antiatherogenic effect of pomegranate juice; however, the direct antioxidant effect requires more mechanistic evidence in an in-vivo situation.
Acknowledgments
We would like to thank Dr. Ephraim Lansky from Rimonest Ltd (Haifa, Israel) for providing the test substances.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abbasi, H., Rezaei, K., & Rashidi, L. (2008). Extraction of essential oils from the seeds of pomegranate using organic solvents and supercritical CO2. Journal of the American Oil Chemists’ Society, 85, 83–89.
- Abbey, M., Nestel, P., & Baghurst, P. A. (1993). Antioxidant vitamins and low-density-lipoprotein oxidation. The American Journal of Clinical Nutrition, 58, 525–532.
- Afaq, F., Saleem, M., Krueger, C. G., Reed, J. D., & Mukhtar, H. (2005). Anthocyanin‐and hydrolyzable tannin‐rich pomegranate fruit extract modulates MAPK and NF‐κB pathways and inhibits skin tumorigenesis in CD‐1 mice. International Journal of Cancer, 113, 423–433.
- Albrecht, M., Jiang, W., Kumi-Diaka, J., Lansky, E. P., Gommersall, L. M., Patel, A., … Campbell, M. J. (2004). Pomegranate extracts potently suppress proliferation, xenograft growth, and invasion of human prostate cancer cells. Journal of Medicinal Food, 7, 274–283.
- Aviram, M., Dornfeld, L., Rosenblat, M., Volkova, N., Kaplan, M., Coleman, R., … Fuhrman, B. (2000). Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: Studies in humans and in atherosclerotic apolipoprotein E–deficient mice. The American Journal of Clinical Nutrition, 71, 1062–1076.
- Aviram, M., Rosenblat, M., Gaitini, D., Nitecki, S., Hoffman, A., Dornfeld, L., … Liker, H. (2004). Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clinical Nutrition, 23, 423–433.
- Aviram, M., Volkova, N., Coleman, R., Dreher, M., Reddy, M. K., Ferreira, D., & Rosenblat, M. (2008). Pomegranate phenolics from the peels, arils, and flowers are antiatherogenic: Studies in vivo in atherosclerotic apolipoprotein E-deficient (E0) mice and in vitro in cultured macrophages and lipoproteins. Journal of Agricultural and Food Chemistry, 56, 1148–1157.
- Azadzoi, K. M., Schulman, R. N., Aviram, M., & Siroky, M. B. (2005). Oxidative stress in arteriogenic erectile dysfunction: Prophylactic role of antioxidants. The Journal of Urology, 174, 386–393.
- Basu, A., & Penugonda, K. (2009). Pomegranate juice: A heart-healthy fruit juice. Nutrition Reviews, 67, 49–56.
- Bhandary, S. K., Kumari, S., Bhat, V. S., Sharmila, K., & Bekal, M. P. (2012). Preliminary phytochemical screening of various extracts of Punica granatum peel, whole fruit and seeds. Journal Health Sciences, 2, 35–38.
- Cáceres, A., Girón, L. M., Alvarado, S. R., & Torres, M. F. (1987). Screening of antimicrobial activity of plants popularly used in Guatemala for the treatment of dermatomucosal diseases. Journal of Ethnopharmacology, 20, 223–237.
- Caligiani, A., Bonzanini, F., Palla, G., Cirlini, M., & Bruni, R. (2010). Characterization of a potential nutraceutical ingredient: Pomegranate (Punica granatum L.) seed oil unsaponifiable fraction. Plant Foods for Human Nutrition, 65, 277–283.
- Chan, K. W., & Ismail, M. (2009). Supercritical carbon dioxide fluid extraction of Hibiscus cannabinus L. seed oil: A potential solvent-free and high antioxidative edible oil. Food Chemistry, 114, 970–975.
- Dadashi, S., Mousazadeh, M., Emam-Djomeh, Z., & Mousavi, S. M. (2013). Pomegranate (Punica granatum L.) seed: A comparative study on biochemical composition and oil physicochemical characteristics. International Journal of Advanced Biological and Biomedical Research, 1, 351–363.
- Davidson, M. H., Maki, K. C., Dicklin, M. R., Feinstein, S. B., Witchger, M., Bell, M., … Aviram, M. (2009). Effects of consumption of pomegranate juice on carotid intima–Media thickness in men and women at moderate risk for coronary heart disease. The American Journal of Cardiology, 104, 936–942.
- De Nigris, F., Williams-Ignarro, S., Botti, C., Sica, V., Ignarro, L. J., & Napoli, C. (2006). Pomegranate juice reduces oxidized low-density lipoprotein downregulation of endothelial nitric oxide synthase in human coronary endothelial cells. Nitric Oxide, 15, 259–263.
- DeWitt, B., III (1987). Improved methodology for the estimation of sulfur dioxide in shrimp. Retrieved from
- El‐Nemr, S., Ismail, I., & Ragab, M. (1990). Chemical composition of juice and seeds of pomegranate fruit. Molecular Nutrition & Food Research, 34, 601–606.
- Esterbauer, H., Gebicki, J., Puhl, H., & Jürgens, G. (1992). The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radical Biology and Medicine, 13, 341–390.
- Fadavi, A., Barzegar, M., & Azizi, M. H. (2006). Determination of fatty acids and total lipid content in oilseed of 25 pomegranates varieties grown in Iran. Journal of Food Composition and Analysis, 19, 676–680.
- Fuhrman, B., & Aviram, M. (2001). Flavonoids protect LDL from oxidation and attenuate atherosclerosis. Current Opinion in Lipidology, 12, 41–48.
- Fuhrman, B., Volkova, N., & Aviram, M. (2005). Pomegranate juice inhibits oxidized LDL uptake and cholesterol biosynthesis in macrophages. The Journal of Nutritional Biochemistry, 16, 570–576.
- Fuhrman, B., Volkova, N., & Aviram, M. (2010). Pomegranate juice polyphenols increase recombinant paraoxonase-1 binding to high-density lipoprotein: Studies in vitro and in diabetic patients. Nutrition, 26, 359–366.
- Gil, M. I., Tomás-Barberán, F. A., Hess-Pierce, B., Holcroft, D. M., & Kader, A. A. (2000). Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. Journal of Agricultural and Food Chemistry, 48, 4581–4589.
- Guo, C., Wei, J., Yang, J., Xu, J., Pang, W., & Jiang, Y. (2008). Pomegranate juice is potentially better than apple juice in improving antioxidant function in elderly subjects. Nutrition Research, 28, 72–77.
- Haber, S. L., Joy, J. K., & Largent, R. (2011). Antioxidant and antiatherogenic effects of pomegranate. American Journal of Health-System Pharmacy, 68, 1302–1305.
- Habibnia, M., Ghavami, M., Ansaripourc, M., & Vosough, S. (2012). Chemical evaluation of oils extracted from five different varieties of Iranian pomegranate seeds. Journal of Food Biosciences and Technology, 2, 35–40.
- Huang, T. H. W., Peng, G., Kota, B. P., Li, G. Q., Yamahara, J., Roufogalis, B. D., & Li, Y. (2005). Pomegranate flower improves cardiac lipid metabolism in a diabetic rat model: Role of lowering circulating lipids. British Journal of Pharmacology, 145, 767–774.
- Ignarro, L. J., Byrns, R. E., Sumi, D., De Nigris, F., & Napoli, C. (2006). Pomegranate juice protects nitric oxide against oxidative destruction and enhances the biological actions of nitric oxide. Nitric Oxide, 15, 93–102.
- Ji, S., Fattahi, A., Raffel, N., Hoffmann, I., Beckmann, M. W., Dittrich, R., & Schrauder, M. (2017). Antioxidant effect of aqueous extract of four plants with therapeutic potential on gynecological diseases; Semen persicae, Leonurus cardiaca, Hedyotis diffusa, and Curcuma zedoaria. European Journal of Medical Research, 22, 50.
- Khateeb, J., Gantman, A., Kreitenberg, A. J., Aviram, M., & Fuhrman, B. (2010). Paraoxonase 1 (PON1) expression in hepatocytes is upregulated by pomegranate polyphenols: A role for PPAR-γ pathway. Atherosclerosis, 208, 119–125.
- Kim, N. D., Mehta, R., Yu, W., Neeman, I., Livney, T., Amichay, A., … Jiang, W. (2002). Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. Breast Cancer Research and Treatment, 71, 203–217.
- Kohno, H., Suzuki, R., Yasui, Y., Hosokawa, M., Miyashita, K., & Tanaka, T. (2004). Pomegranate seed oil rich in conjugated linolenic acid suppresses chemically induced colon carcinogenesis in rats. Cancer Science, 95, 481–486.
- Konishi, Y., Hitomi, Y., & Yoshioka, E. (2004). Intestinal absorption of p-coumaric and gallic acids in rats after oral administration. Journal of Agricultural and Food Chemistry, 52, 2527–2532.
- Lad, V., & Frawley, D. (1986). The Yoga of herbs. Twin Lakes, WI: Lotus Press.
- Lansky, E. P., & Newman, R. A. (2007). Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. Journal of Ethnopharmacology, 109, 177–206.
- Liu, G., Xu, X., Gong, Y., He, L., & Gao, Y. (2012). Effects of supercritical CO 2 extraction parameters on chemical composition and free radical-scavenging activity of pomegranate (Punica granatum L.) seed oil. Food and Bioproducts Processing, 90, 573–578.
- Liu, G., Xu, X., Hao, Q., & Gao, Y. (2009). Supercritical CO 2 extraction optimization of pomegranate (Punica granatum L.) seed oil using response surface methodology. LWT-Food Science and Technology, 42, 1491–1495.
- Liu, W., Fu, Y.-J., Zu, Y.-G., Tong, M.-H., Wu, N., Liu, X.-L., & Zhang, S. (2009). Supercritical carbon dioxide extraction of seed oil from Opuntia dillenii Haw. and its antioxidant activity. Food Chemistry, 114, 334–339.
- Mahajan, D., Bhat, Z., & Kumar, S. (2015). Pomegranate (Punica granatum) rind extract as a novel preservative in cheese. Food Bioscience, 12, 47–53.
- Mori-Okamoto, J., Otawara-Hamamoto, Y., Yamato, H., & Yoshimura, H. (2004). Pomegranate extract improves a depressive state and bone properties in menopausal syndrome model ovariectomized mice. Journal of Ethnopharmacology, 92, 93–101.
- Nasiri, E., Hesari, J., Shekarforoush, S., & Kooshesh, S. (2016). Effect of aqueous extract of myrtle leaves (Myrtus communis) on quality changes of farmed gutted rainbow trout (Oncorhynchus mykiss) during chilled (4 ±1°C) storage. Iranian Scientific Fisheries Journal, 25, 1–14.
- Nasiri, E., Moosavi-Nasab, M., Shekarforoush, S., & Golmakani, M. (2015). The effects of Zataria multiflora on inhibition of polyphenoloxidase and melanosis formation in shrimp (Litopenaeus vannamei. Isfj, 23, 109–118.
- Naveena, B., Sen, A., Vaithiyanathan, S., Babji, Y., & Kondaiah, N. (2008). Comparative efficacy of pomegranate juice, pomegranate rind powder extract and BHT as antioxidants in cooked chicken patties. Meat Science, 80, 1304–1308.
- Naveena, B., Sen, A., Vaithiyanathan, S., Muthukumar, M., & Babji, Y. (2007). Microwave cooking properties of chicken patties containing honey and vitamin-C. Journal of Food Science and Technology, 44, 505–508.
- Orhan, I., Aydin, A., Çölkesen, A., Sener, B., & Isimer, A. (2003). Free radical scavenging activities of some edible fruit seeds. Pharmaceutical Biology, 41, 163–165.
- Özgül-Yücel, S. (2005). Determination of conjugated linolenic acid content of selected oil seeds grown in Turkey. Journal of the American Oil Chemists’ Society, 82, 893–897.
- Pirillo, A., Norata, G. D., & Catapano, A. L. (2013). LOX-1, OxLDL, and atherosclerosis. Mediators of Inflammation, 2013. doi:10.1155/2013/152786
- Rahimi, H. R., Arastoo, M., & Ostad, S. N. (2012). A comprehensive review of Punica granatum (pomegranate) properties in toxicological, pharmacological, cellular and molecular biology researches. Iranian Journal of Pharmaceutical Research, 11, 385–400.
- Rosenblat, M., & Aviram, M. (2006). Antioxidative properties of pomegranate: In vitro studies. In N. P. Seeram, R. N. Schulman & D. Heber (Eds.), Pomegranates: Ancient roots to modern medicine (pp. 31–43).
- Saxena, A., & Vikram, N. K. (2004). Role of selected Indian plants in management of type 2 diabetes: A review. The Journal of Alternative & Complementary Medicine, 10, 369–378.
- Schubert, S. Y., Lansky, E. P., & Neeman, I. (1999). Antioxidant and eicosanoid enzyme inhibition properties of pomegranate seed oil and fermented juice flavonoids. Journal of Ethnopharmacology, 66, 11–17.
- Seeram, N. P., Adams, L. S., Henning, S. M., Niu, Y., Zhang, Y., Nair, M. G., & Heber, D. (2005). In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. The Journal of Nutritional Biochemistry, 16, 360–367.
- Seeram, N. P., Aviram, M., Zhang, Y., Henning, S. M., Feng, L., Dreher, M., & Heber, D. (2008). Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. Journal of Agricultural and Food Chemistry, 56, 1415–1422.
- Seeram, N. P., Lee, R., & Heber, D. (2004). Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clinica Chimica Acta, 348, 63–68.
- Sezer, E. D., Akçay, Y. D., Ilanbey, B., Yıldırım, H. K., & Sözmen, E. Y. (2007). Pomegranate wine has greater protection capacity than red wine on low-density lipoprotein oxidation. Journal of Medicinal Food, 10, 371–374.
- Shahrzad, S., & Bitsch, I. (1998). Determination of gallic acid and its metabolites in human plasma and urine by high-performance liquid chromatography. Journal of Chromatography B: Biomedical Sciences and Applications, 705, 87–95.
- Singh, R., Chidambara Murthy, K., & Jayaprakasha, G. (2002). Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. Journal of Agricultural and Food Chemistry, 50, 81–86.
- Topuz, O. K., Yerlikaya, P., Ucak, I., Gumus, B., & Büyükbenli, H. A. (2014). Effects of olive oil and olive oil–Pomegranate juice sauces on chemical, oxidative and sensorial quality of marinated anchovy. Food Chemistry, 154, 63–70.
- Ünalan, U., Dalgaard, P., & Korel, F. (2011). Effect of pomegranate (Punica granutum) and rosemary (Rosmarinus officinalis L.) extracts on shelf-life for chilled Greenland halibut (Reinhardtius hippoglossoides) fillets in modified atmosphere packaging at 2ºC. Paper presented at the International Food Congress Novel Approaches In Food Industry, Izmir, Turkey.
- Vaithiyanathan, S., Naveena, B., Muthukumar, M., Girish, P., & Kondaiah, N. (2011). Effect of dipping in pomegranate (Punica granatum) fruit juice phenolic solution on the shelf life of chicken meat under refrigerated storage (4 C). Meat Science, 88, 409–414.
- Viuda-Martos, M., Ruiz-Navajas, Y., Fernández-López, J., Sendra, E., Sayas-Barberá, E., & Pérez-Álvarez, J. A. (2011). Antioxidant properties of pomegranate (Punica granatum L.) bagasses obtained as co-product in the juice extraction. Food Research International, 44, 1217–1223.
- Yamasaki, M., Kitagawa, T., Koyanagi, N., Chujo, H., Maeda, H., Kohno-Murase, J., … Yamada, K. (2006). Dietary effect of pomegranate seed oil on immune function and lipid metabolism in mice. Nutrition, 22, 54–59.
- Zahin, M., Aqil, F., & Ahmad, I. (2010). Broad spectrum antimutagenic activity of antioxidant active fraction of Punica granatum L. peel extracts. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 703, 99–107.
- Zhang, H., Chen, H., Yang, D., Chen, W., & Ying, X. (2014). In situ intestinal absorption of ellagic acid in rats. Chinese Journal of Hospital Pharmacy, 14, 008.