ABSTRACT
Detection of biofilms on food-contact surfaces is essential for establishing sanitization procedures and avoiding microbial contamination of food products. The objective of our study was to develop a peroxide biodetector, to detect and reveal the presence of biofilms. A positive reaction was observed when biodetector bubbles were formed on stainless steel and polypropylene surfaces. A range of microorganisms able to form biofilms was evaluated. Catalase-positive bacteria: Staphylococcus aureus, Escherichia coli, Listeria monocytogenes, Pseudomonas aeruginosa, Salmonella enterica ser. Typhimurium and Cronobacter sakazakii were employed as well as the catalase-negative bacterium: Lactobacillus brevis. With the exception of C. sakazakii, all catalase-positive foodborne pathogens forming the biofilms showed a positive detection by the biodetector, being 104 CFU cm−2 the minimum microbial load detected. The strongest positive reaction was for P. aeruginosa. Results demonstrated the potential of this biodetector to detect biofilms, particularly when used as a tool in the food industry.
RESUMEN
La detección de biofilms en superficies de contacto con alimentos es, hoy en día, esencial para establecer procedimientos de limpieza y desinfección eficaces y evitar la contaminación microbiana de productos alimenticios. El objetivo del presente estudio fue el desarrollo de un biodetector a base de peróxido, para detectar y revelar la presencia de biofilms mediante la formación de burbujas en superficies de acero inoxidable y polipropileno. Diferentes microorganismos fueron empleados en el estudio, entre ellos, bacterias catalasa positivas (Staphylococcus aureus, Escherichia coli, Listeria monocytogenes, Pseudomonas aeruginosa, Salmonella enterica ser. Typhimurium y Cronobacter sakazakii) y una bacteria catalasa negativa (Lactobacillus brevis). Con la excepción de C. sakazakii, el resto de patógenos mostraron una detección positiva por el biodetector, siendo 104 CFU cm−2 la mínima carga microbiana detectada. La reacción positiva más abundante fue para P. aeruginosa. Los resultados obtenidos demostraron el gran potencial que tiene el biodetector para la detección de biofilms, especialmente cuando éste se usa como herramienta en la industria alimentaria.
PALABRAS CLAVE:
Introduction
Contaminated food-contact surfaces may allow bacteria to attach themselves and subsequently develop biofilms, which become a potential source of cross-contamination of food products (Hood & Zottola, Citation1995; Lee Wong, Citation1998; Myszka & Czaczyk, Citation2011). A biofilm is a community of microorganisms attached to a surface that produces an amorphous three-dimensional matrix of extracellular polymeric substances (EPS). They simultaneously serve as a sticking vehicle to attach different microorganisms (LeChevallier, Cawthon, & Lee, Citation1988; Stepanović, Cirković, Ranin, & Svabić-Vlahović, Citation2004; Whittenbury, Citation1964). An exceptional biofilm characteristic is that once it is formed on food or food-processing facility surfaces, its complete removal using common cleaning and sanitation procedures is very difficult (Baumann, Martin, & Feng, Citation2009; Torlak & Sert, Citation2013). This fact poses a serious concern to food safety as pathogenic microorganisms can be involved in the development of biofilms in food and on food-contact surfaces, and therefore biofilm cells can be released resulting in a cross-contamination to food products (Shi & Zhu, Citation2009; Simões, Simões, & Vieira, Citation2010).
Listeria monocytogenes and other pathogens readily produce biofilms, which helps them to survive for prolonged periods in food production plants (Swaminathan & Gerner-Smidt, Citation2007; Tompkin, Citation2002). To prevent biofilm formation, sanitization programmes that target mature biofilms for maximal bacterial inactivation should be established (Yang, Kendall, Medeiros, & Sofos, Citation2009). Current available methods and procedures are insufficient to achieve an efficient control of the different kinds of microorganisms found not only in the food itself, but also in the food production process, the processing equipment and related environments (Carlton, Noordman, Biswas, de Meester, & Loessner, Citation2005). In fact, reported outbreaks of E. coli O157:H7 and Salmonella spp. have been related to cross-contamination and subsequent biofilm formation on lettuce, spinach, and cantaloupe melons (Annous, Fratamico, & Smith, Citation2009), indicating that there is a need for better methods to control contamination.
In general, the research regarding biofilms has been centred on monospecies and pure cultures. However, biofilms are formed by multiple microbial species in natural environments (Manuzon & Wang, Citation2007; Montañez-Izquierdo, Salas-Vázquez, & Rodríguez-Jerez, Citation2012; Moons, Michiels, & Aertsen, Citation2009; Percival, Walker, & Hunter, Citation2000). Different studies have outlined the significance of biofilms formed by mixed species for the food industry (Jahid & Ha, Citation2014; Percival et al., Citation2000). The importance for this industry is attributed to the existence of interspecies interactions that might enhance not only the survival of pathogens to sanitizers, disinfectants, UV light, or desiccation, but also its persistence on food-contact surfaces (Lee et al., Citation2014). In a study conducted by Jahid, Han, Srey, and Ha (Citation2014b), it was demonstrated that biofilms formed by S. Typhimurium and cultivable microflora from lettuce became more resistant to UV-C treatments than biofilms formed with mono-cultures. In another study, it was reported how mixed biofilms of L. monocytogenes, Pseudomonas fluorescens, Serratia proteamaculans, and Shewanella baltica formed on stainless steel surfaces produced more EPS and were more resistant to desiccation (Alavi & Hansen, Citation2013).
Until now, identifying the type of microorganisms involved as contaminating agents and establishing the source and extent of contamination by biofilms have been conducted using enumeration techniques. The different methods employed range from the most conventional techniques to the most recent developments. Some traditional cultivation techniques such as swabbing, rinsing, and agar contact methods have been used to quantify the microorganisms on external and internal surfaces (Kumar & Anand, Citation1998). These methods can present some standardization problems when collecting samples from different surfaces in the food industry. For this reason, a surface sensor named SCH (Hygiene Control Sensor, Premiumlab, Sant Boi del Llobregat, Spain) was developed to monitor the biological contamination of surfaces and offer an alternative to traditional methods when analysing product safety and quality. However, the detection of pathogens on food-contact surfaces is mainly founded on traditional microbiological culture methods that can take considerable time to complete (Branda, Vik, Friedman, & Kolter, Citation2005). This is problematic because reducing the time required for the detection and confirmation of microbial contamination is highly important for the food industry.
Methods based on microscopy, particularly direct epifluorescence microscopy (DEM) and scanning electron microscopy (SEM) of surfaces, have attracted significant attention in the study of biofilms (Notermans, Nauta, Jansen, Jouve, & Mead, Citation1998). DEM with digital image analysis is a rapid method used to enumerate microorganisms. This technique is able to distinguish between viable and nonviable cells (Holah, Betts, & Thorpe, Citation1989; Lunau, Lemke, Walther, Martens-Habbena, & Simon, Citation2005; Pettipher & Rodrigues, Citation1982). Moreover, DEM approaches provide the opportunity to visualize bacteria on the substrate in the state of adherence (Hannig et al., Citation2007). A strong linear correlation between the counts by DEM and conventional culture has been observed (Montañez-Izquierdo et al., Citation2012). However, when microorganisms are exposed to adverse conditions, the correlation for both methods decreases significantly. This reduction is attributed to the presence of a bacterial stress response that cannot be detected by cultivation (Fuster-Valls, Hernández-Herrero, Marín-de-Matero, & Rodríguez-Jerez, Citation2008).
For the food industry, it is vital to obtain rapid results of the different microbiological analysis of food-contact surfaces in order to know, not only their hygienic state, but also to be able to take decisions. The development of a product that can detect the presence of biofilms in a short time is a powerful tool to ensure the safety of foods.
The objective of this study was to consider the potential for developing a biodetector for a direct biofilm detection on surfaces of catalase-positive bacteria. With this purpose, an evaluation of the environmental conditions to ensure the development of biofilms on stainless steel and polypropylene surfaces was performed. Furthermore, different formulas to obtain an efficient biofilm biodetector were evaluated.
Materials and methods
Test surfaces
Two materials were assayed: different-sized 1 mm thick stainless steel 304 grade 2B coupons (first area: diameter disc of 2 cm, second area: 5 × 5 cm, third area: 20 × 20 cm) and different-sized 0.4 mm thick polypropylene coupons (first area: 5 × 5 cm, second area: 20 × 20 cm). In both cases and prior to being used, the surfaces were cleaned and disinfected in accordance with ISO 13697:2001 (Anonymous, Citation2002). First, a wash was performed with a surfactant (Dipol Bac Stop, D.I.S. DINO S.L., Madrid, Spain). Second, a solution of 2-propanol in water (70%) was applied to eliminate the residue of the surfactant (Panreac Química, Barcelona, Spain). The stainless steel surfaces were further sterilized by autoclave at 121°C for 15 min. The polypropylene coupons were sterilized by placing them in a laminar flux sterile cabinet (Telstar, Terrassa, Spain) and exposing them to the antibacterial action and evaporation of 2-propanol for 30 min (Anonymous, Citation2007).
Test organisms
Frozen-dried cultures of different strains of microorganisms were employed in this study: Staphylococcus aureus ATCC 6538, Escherichia coli CECT 515, Listeria monocytogenes CCUG 15526, Pseudomonas aeruginosa ATCC 15442, Salmonella enterica ser. Typhimurium CCUG 29478, Cronobacter sakazakii ATCC BAA-894 and Lactobacillus brevis CECT 216.
Bacterial cultures for the tests
Bacterial cultures were recovered using buffered peptone water (BPW, Buffered Peptone Water; bioMérieux®, Marcy l’Etoile, France) and incubated at 37°C for 18–24 h. After the incubation period, the different bacterial cultures were streaked on TSA (Tryptic Soy Agar; Biokar Diagnostics, Beauvais, France) and incubated at 30°C for 24 h. Only L. brevis was rehydrated in MRS broth (Man-Rogosa-Sharpe Broth, Oxoid, UK), incubated at 30°C for 48 h and finally streaked on MRS agar (30°C for 24 h). From each strain, isolated colonies were selected and prepared as stock cultures in TSA or MRS agar slants. These slants were stored as working cultures at 4°C for a maximum period of 30 days.
Each stock culture in slants was subcultured prior to preparing the bacterial inoculums. This procedure was made (i) in 9 ml of BPW in the preliminary step before the biofilm formation, and (ii) in 9 ml of TSB (Tryptic Soy Broth; Biokar Diagnostics, Beauvais, France) in the final step during biofilm formation. The incubation periods were selected taking into account the optimal temperature and times for each microorganism. Incubations were performed at 37°C for 18–20 h in order to reach the stationary phase. However, for L. monocytogenes the incubation period was set at 20–22 h. In the case of L. brevis, 5 ml of MRS broth was used to prepare the inoculum, which was incubated at 30°C for 22–24 h. From each bacterial culture obtained, decimal dilutions were conducted using TSS (Tryptone Saline Solution) (1 g of tryptone [Becton Dickinson and Co.], 8.5 g of sodium chloride [Panreac Química]) and 1000 ml of deionized water (pH 7.0 ± 0.2)).
Biofilm formation
To ensure biofilm formation, sterile coupons of stainless steel and polypropylene were placed in sterile Petri dishes. Each coupon was inoculated with 100 µl of TSS containing a concentration of 104 CFU ml−1 cells of corresponding bacterial inoculums described above. The surfaces inoculated were placed in Petri dishes and then placed into a humidified chamber and incubated at room temperature (22–24°C) for 48 or 72 h (Fuster-Valls et al., Citation2008) under five different sets of conditions to form biofilms (1) Static condition. Without renewal growth culture medium. The bacterial inoculums on the surfaces were not manipulated until the end of the incubation period. (2) With two washes and the addition of culture medium. The washing steps were performed with 5 ml of physiologic saline solution (PSS: 8.5 g of sodium chloride in 1000 ml of deionized water; pH 7.0 ± 0.2), followed by an inoculation of 100 µl of sterile TSS or MRS broth. This step was performed at 24 and 48 h. (3) Three washes and the addition of culture medium. The washings were performed with 5 ml of PSS, followed by an inoculation of 100 µl of sterile TSS or MRS broth. This step was performed at 3, 24, and 48 h. (4) With addition of 100 µl of sterile TSS or MRS broth in order to just renew the nutrients. This procedure was performed without the PSS wash and was performed after 24 h. (5) With addition of 50 µl of sterile TSS or MRS broth with the same objective mentioned above, the renewal of nutrients. This procedure was performed without the PSS wash and after 24 h of the incubation.
Furthermore, with the aim of ensuring a positive control for every condition, surfaces were inoculated with 400 µl of a solution with 104 CFU ml−1 and 400 µl of sterile TSS in order to have biofilms that occupied a wide surface area. Surfaces were subsequently placed in a saturated humidified environment for 72 h. After 24 and 48 h, surfaces were washed with 5 ml of PSS followed by an inoculation of 800 µl of sterile TSS or MRS broth.
Biodetector
Different formulas for the development of the biodetector were evaluated: (i) Solution with hydrogen peroxide; (ii) Solution with hydrogen peroxide and crystal violet dye; (iii) Solution with hydrogen peroxide, phosphonates (<5%), surfactans (5–15%), bleaching agents (5–15%), and an orange colorant.
Biofilm detection using epifluorescent microscopy and the biodetector
After the incubation time, biofilms that formed on surfaces were washed with 5 ml of PSS and analysed by both, direct epifluorescent microscopy and the biodetector.
Epifluorescent microscopy
After surfaces were washed, vital staining was employed with 20 µl of the kit LIVE/DEAD® BacLightTM (L13152; Molecular Probes Inc., Oregon, USA). This kit is composed of two fluorescent dyes of nucleic acids, SYTO®9 and propidium iodide (PI). SYTO®9, which penetrate cells with either intact or injured membranes. In contrast, PI penetrates only injured membrane cells and reduces the dye SYTO®9. Therefore, with the simultaneous application of these two dyes in appropriate proportions viable cells with intact membranes show up in fluorescent green and dead or injured cells show up in fluorescent red.
After applying the stain, the samples were incubated at room temperature for 15 min in the dark according to the manufacturer’s instructions. Readings were performed with an epifluorescent microscope Olympus BX51/BX52 (Olympus, Tokyo, Japan) equipped with a mercury lamp of 100 W (USH-103OL, Olympus), a double pass filter (U-M51004 F/R – V2, Olympus), and a digital camera DP50-CU (Olympus). Stained samples were observed with 20× and 40× objectives.
Detecting biofilms
After the corresponding incubation periods to form biofilms of the different bacterial strains, the different hydrogen peroxide formulas were evaluated. The evaluation was performed by spraying 100 µl of the biodetector on the surface to be analysed and the result was observed after 10 min. A reaction was considered positive when a formation of bubbles was observed from the biofilms formed on stainless steel or polypropylene surfaces. The bubbles are caused by an enzymatic reaction that releases oxygen when catalase enzyme from microorganisms is present.
Statistical data analysis
All tests were performed in three separate experiments by triplicate (n = 9). Images obtained by DEM were analysed by using the analySIS Auto 3.2 software (Soft Imaging System GMBH, Münster, Germany), and the obtained counts were converted into decimal logarithmic values to nearly match the assumption of a normal distribution. The SAS® v9.1.3.4. statistical software package (SAS Inst. Inc., Cary, N.C., U.S.A.) was employed to evaluate differences between the conditions for biofilm formation by analysing the variance (ANOVA). A p value of <0.05 was considered to be significant.
Results and discussion
Detection of biofilms using DEM and the different biodetector formulas
From the data obtained, it was observed that biofilms of E. coli, P. aeruginosa, and S. Typhimurium were intensely formed after three washes ( and ), indicating that the bacterial density was clearly greater in this condition. Nevertheless, the count of microorganisms obtained by the image analysis system of biofilms formed with three washes compared from those formed with two washes did not show significant differences (P > 0.05). L. monocytogenes did not develop biofilms after the first washing step and attachment was observed in the static conditions, showing that this pathogen might need more time for its initial adhesion to the stainless steel. Our results are in accordance with those described by Chae and Schraft (Citation2000), showing that a minimum incubation period of 3 h was needed for the adhesion of this pathogen to glass, suggesting that there is also an optimum incubation period for its adhesion to stainless steel and polypropylene. The rest of the bacteria, S. aureus, L. brevis, and C. sakazakii showed a low attachment capacity compared with L. monocytogenes in this study. These microorganisms probably needed more incubation periods, the presence of more active microorganisms to develop the polymeric structure and other environmental factors that have an influence on the development of biofilms (Garrett, Bhakoo, & Zhang, Citation2008; Van Houdt & Michiels, Citation2010).
Figure 1. Biofilms of: (A) L. brevis, (B) S. Typhimurium, and (C) C. sakazakii. Biofilms formed on stainless steel, without washes (A-1, B-1, and C-1), and with three washes (A-2, B-2, and C-2). Epifluorescent images obtained with LIVE/DEAD® stain. Viable cells stained in green colour and not viable cells in red. (All figures at 40× magnification).
Figura 1. Biofilms de: (A) L. brevis, (B) S. Typhimurium y (C) C. sakazakii. Las biopelículas se formaron en acero inoxidable, sin enjuagues (A-1, B-1, y C-1), y con tres enjuagues (A-2, B-2, y C-2). Las imágenes epifluorescentes se obtuvieron mediante la aplicación del colorante LIVE/DEAD®. Las células viables se tiñeron de verde y las no viables de rojo. (Todas las figuras aparecen con un aumento de 40×.)
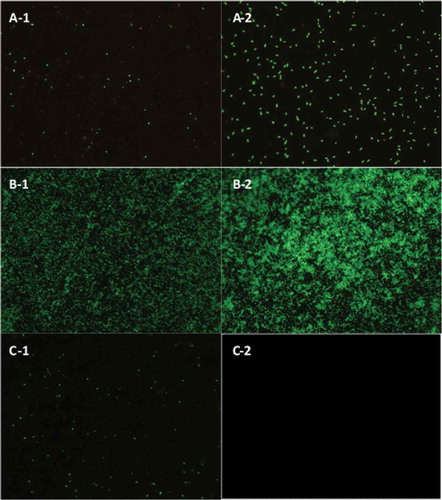
Figure 2. Biofilms of: (A) S. aureus, E. coli (B), L. monocytogenes (C), and P. aeruginosa (D). Biofilms formed on stainless steel, without washes (A-1, B-1, C-1, and D-1), and with three washes (A-2, B-2, C-3, and D-2). Epifluorescent images obtained with LIVE/DEAD® stain. Viable cells stained in green colour and not viable cells in red. (All figures at 40× magnification).
Figura 2. Biofilms de: (A) S. aureus, E. coli (B), L. monocytogenes (C), y P. aeruginosa (D). Los biofilms se formaron en acero inoxidable, sin enjuagues (A-1, B-1, C-1, y D-1), y con tres enjuagues (A-2, B-2, C-3, y D-2). Las imágenes de epifluorescencia directa se obtuvieron mediante la aplicación del colorante LIVE/DEAD®. Las células viables se tiñeron de verde y las no viables de rojo. (Todas las figuras aparecen con un aumento de 40×.)
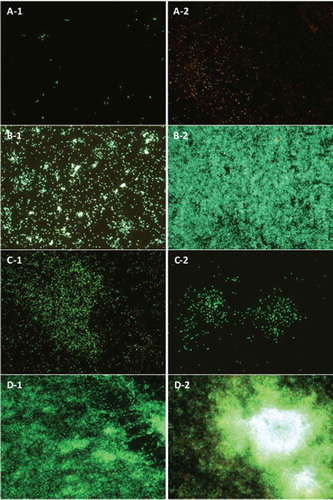
When surfaces were washed twice, the results were very similar to those obtained with three washes for E. coli, P. aeruginosa, and S. Typhimurium. These microorganisms developed more intense biofilms with a higher bacterial density and a higher microbial count. Significant differences (P < 0.05) were found on the counts when compared to those that were incubated in static conditions without the renewal of nutrients. Previous reports have shown the importance of a constant flow of nutrients for the development of biofilms that is vital in the early stages of their formation (Myszka & Czaczyk, Citation2011; Simões et al., Citation2010) Furthermore, under these conditions increased biofilm formation for S. aureus, L. brevis, and C. sakazakii was observed. Our results reveal that even under adverse conditions, a catalase-positive microorganism such as S. aureus represent a resistant bacterium to common hygienic procedures (Ríos-Castillo, González-Rivas, & Rodríguez-Jerez, Citation2017) and is able to form biofilms (Di Ciccio et al., Citation2015; Fuster-Valls et al., Citation2008). The difference of biofilm formation obtained in this experiment in comparison with the previous experiment can be justified because in the previous experiment the first wash was performed after 24 h, whereas in this experimental design the first wash was performed after an incubation period of 3 days. Therefore, it might be concluded that a long incubation period before the first wash is necessary to increase the biofilm capacity of these microorganisms. Similar results were found in a study conducted by Wang et al. (Citation2017) on where it was demonstrated that the increase in biofilm formation was time-dependent before 5-days incubation period, obtaining nearly 10.5 log CFU cm−2 cells of Enterobacter cloacae on stainless steel when incubated under long-term (7 days) growth scenario.
L. monocytogenes developed a high-density biofilm when the static condition was applied and behaved differently to the rest of microorganisms. However, biofilms obtained for E. coli, P. aeruginosa, and S. Typhimurium were denser after two washes, the first one starting after 24 h of incubation.
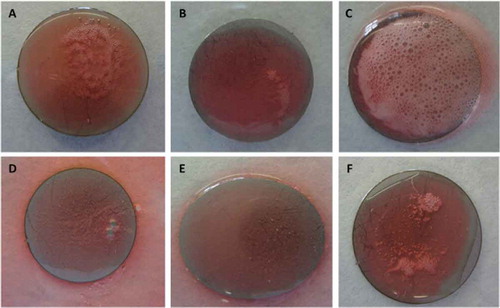
The strongest biofilm formers obtained were then employed for the evaluation of the biofilm detection capacity of the different biodetectors formulated. When the solution with just hydrogen peroxide was applied on E. coli, P. aeruginosa, and S. Typhimurium biofilms, bubble formations were detected, and it was considered as a positive reaction to the presence of biofilms. The election of hydrogen peroxide as an indicator of the presence of biofilms in this study was justified by the positive activity of this compound in different types of bacteria commonly found in food-processing environments reported by Elkins, Hassett, Stewart, Schweizer, and MCDermott (Citation1999); Christensen, Trønnes, Vollan, Smidsrød, and Bakke (Citation1990); and Ríos-Castillo et al. (Citation2017). When the solution of hydrogen peroxide that contained crystal violet stain was applied to the surfaces (), the biofilm area stained blue and an intense bubble formation was observed, indicating that biofilms are more visual when colorants are included in the formula. Finally, when the solution with the hydrogen peroxide and the colorant was applied in a gel form, an even stronger positive reaction was detected, with an abundant bubble formation on the surfaces that contained the biofilms from E. coli and P. aeruginosa (). Surfaces with biofilms from S. aureus, S. Typhimurium, and L. monocytogenes showed a weaker reaction. However, surfaces with biofilm from C. sakazakii did not show any positive reaction. This could be explained by the fact that, according to previous studies, C. sakazakii cells imbedded within biofilms have a high resistance to stress conditions (Yan & Gurtler, Citation2014) and is affected by atmospheric relative humidity (Beuchat et al., Citation2009). Therefore, may be for this bacterium, a larger amount of hydrogen peroxide is necessary to show a positive reaction. Surfaces with biofilms of L. brevis did not show any positive reaction because this microorganism is catalase negative (Whittenbury, Citation1964) and no positive action was expected. In food industry environment, the non-detection of negative catalase microorganisms would not be a problem because biofilms are formed by mixed species (Colagiorgi et al., Citation2017; Elias & Banin, Citation2012; Phillips, Citation2016) and therefore the use of the biodetector would show microbial activity. Biofilms formed by mixed species constitutes a general adaptation of foodborne pathogens and microbiota associated to the food-processing environment for extended survival in their food niche (Jahid & Ha, Citation2014).
Figure 3. Detection of P. aeruginosa biofilms by (A) solution with hydrogen peroxide, (B-1) crystal violet, and (B-2) solution with hydrogen peroxide and crystal violet.
Figura 3. Detección de biofilms de P. aeruginosa biofilms mediante: (A) solución con peróxido de hidrógeno, (B-1) violeta cristal, y (B-2) solución con peróxido de hidrógeno y violeta cristal.

Figure 4. Reactions after the application of the solution with hydrogen peroxide, phosphonates, surfactants, and bleaching agents on stainless steel surfaces with biofilms of (A) E. coli, (B) S. aureus, (C) P. aeruginosa, (D) S. Typhimurium, (E) C. sakazakii, and (F) L. monocytogenes.
Figura 4. Reacciones después de la aplicación de la solución con peróxido de hidrógeno, fosfonatos, surfactantes, y agentes blanqueadores en las superficies de acero inoxidable con los biofilms de (A) E. coli, (B) S. aureus, (C) P. aeruginosa, (D) S. Typhimurium, (E) C. sakazakii, y (F) L. monocytogenes.
As the formula that showed the most visual results was the latest, it was further selected for the development of the industrial product. By adapting the classic hydrogen peroxide detection method, a novel gel has been formulated for its direct use in the food industry. It shows bacterial presence on food-contact surfaces, demonstrating its capacity for being an innovative and effective tool for hygiene monitoring. In order to screen its detection power, a spray was applied to surfaces in a flat position on were biofilms were formed. As shown in , positive reactions were very clear and there was an intense bubble formation. When the surface was placed in an upright position, a bubble formation was observed after the biodetector was sprayed onto it (). They were laid flat to be able to mark the positive area clearly. Furthermore, for practical use in the food industry as an indicator of biofilm presence, the biodetector was developed to be easy to clean after its use. For this reason, the biodetector was formulated for being highly water soluble, which aids in rinsing and does not leave stain or residues on surfaces.
Figure 5. Biofilms on stainless steel coupons detected after the spray application of the solution with hydrogen peroxide, phosphonates, surfactants, and bleaching agents of (A) E. coli, (B) P. aeruginosa, and (C) L. brevis, placed in a flat position.
Figura 5. Biofilms en cupones de acero inoxidable detectadas después de la aplicación mediante rociado de la solución con peróxido de hidrógeno, fosfonatos, surfactantes y agentes blanqueadores de (A) E. coli, (B) P. aeruginosa, y (C) L. brevis, colocadas en posición plana.
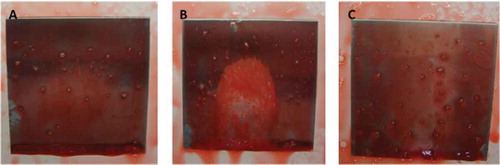
Figure 6. Reactions after the spray application of the solution with hydrogen peroxide, phosphonates, surfactants, and bleaching agents on stainless steel surfaces for the detection of biofilms from (A) E. coli, (B) P. aeruginosa, and (C) L. brevis, formed with different inoculum volumes (25 µl, 50 µl, 100 µl, and 200 µl).
Figura 6. Reacciones después de la aplicación mediante rociado de la solución con peróxido de hidrógeno, fosfonatos, surfactantes, y agentes blanqueadores en las superficies de acero inoxidable para la detección de biofilms generados por (A) E. coli, (B) P. aeruginosa, y (C) L. brevis, formadas con distintos volúmenes de inóculo (25 µl, 50 µl, 100 µl, y 200°µl).
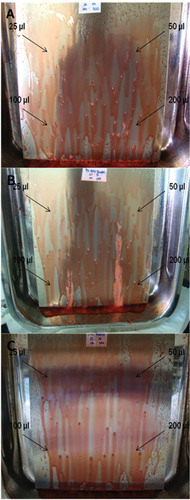
The inoculum volume not only affected the biofilm formation, but also caused a positive reaction after the biodetector was applied. On comparing the positive reaction of the biofilms formed by E. coli and P. aeruginosa and considering the inoculum volumes, 25 µl, 50 µl, 100 µl, and 200 µl (), 25 µl showed no positive reaction for any microorganism. For L. brevis a negative reaction was detected in all cases, as was expected.
Table 1. Counts of microorganisms by microscopic epifluorescent assay on the biofilms formed with inoculum of 25 µl, 50 µl, 100 µl, and 200 µl. Results expressed as log10 CFU cm−2.
Tabla 1. Recuentos de microorganismos detectados en los biofilms formados con inóculos de 25 µl, 50 µl, 100 µl, y 200 µl realizados por ensayo microscópico epifluorescente. Los resultados son expresados en log10 CFU cm−2.
Biodetector final formula
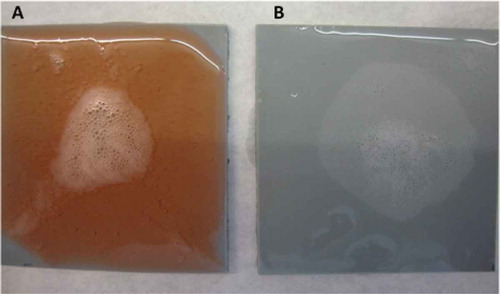
From the three formulas tested, the one that gave the best results, as the contrast was clearer was the solution that contained hydrogen peroxide, phosphonates, surfactans, and bleaching agents. When this formula was used on polypropylene surfaces with P. aeruginosa biofilms, a strong positive reaction was observed after 5 s. This reaction was maintained for more than 5 min. Not only did the orange colour contrast well with the white bubbles, but it also did not stain the material employed (). When the use of this solution was compared with a solution of hydrogen peroxide, the solution was shown to have a poor contrast. The use of the developed biofinder, however, maintained the foam formed for a long time and ensured better detection ().
Figure 7. Reaction from P. aeruginosa biofilms on polypropylene surfaces using the solution with hydrogen peroxide, phosphonates, surfactants, and bleaching agents after (A) zero, (B) 5 s, and (C) 5 min.
Figura 7. Reacción de biofilms de P. aeruginosa en superficies de polipropileno, usando la solución con peróxido de hidrógeno, fosfonatos, surfactantes, y agentes blanqueadores después de (A) 0, (B) 5 segundos, y (C) 5 minutos.

Figure 8. Reaction from P. aeruginosa biofilms on polypropylene surfaces using (A) solution with hydrogen peroxide, phosphonates, surfactants, and bleaching agents, and (B) solution with hydrogen peroxide.
Figura 8. Reacción de los biofilms de P. aeruginosa en superficies de polipropileno usando (A) solución con peróxido de hidrógeno, fosfonatos, surfactantes, y agentes blanqueadores, y (B) solución con peróxido de hidrógeno.
Processed products can be contaminated through biofilms from food-processing environments, increasing disease transmission and reducing the shelf life of food products (Chmielewski & Frank, Citation2003). In fact, the significance of biofilms in food-processing environments is still not well understood owing to a lack of methodology that allows the direct observation of biofilms in these environments. The development of a system that enables a rapid and visual biofilm detection on food-contact surfaces is highly interesting for controlling the hygienic state of these environments in food industries.
As it has been outlined before, biofilms in natural environments are defined as microbial communities formed of multiple species, including pathogens. In fact, some studies carried on the microbial composition of biofilms on food-processing surfaces have revealed that those which are present on processing line surfaces are established by various microorganisms (Bagge-Ravn et al., Citation2003; Gunduz & Tuncel, Citation2006; Guobjornsdottir, Einarsson, & Thorkelsson, Citation2005). If food-contact surfaces are contaminated with biofilms, numerous microbial species will be involved in their formation, including both negative and positive catalase producers (Sharma & Anand, Citation2002). For that reason, the application of the biodetector on those surfaces will show an intense bubble formation, and therefore a positive result revealing microbial activity, even when negative catalase producers are forming the biofilm, as catalase producers are also present. Furthermore, the biodetector can be used not only to reveal microbial activity, but also as a tool for the food-processing line workers responsible for making decisions in regard to cleaning procedures.
Detection limit
The minimum microbial load for a positive reaction was 104 CFU cm−2, a concentration obtained when biofilms were formed after 24–48 h on surfaces with humid environmental conditions. Traditional swabbing or contact plate does not easily detect this number of microorganisms and in many cases, very low recovery is obtained (Nivens, Co, & Franklin, Citation2009). Those false negative results can be explained by the fact that, when using these techniques, the EPS of the biofilm is not being broken and thus the real number of bacteria is not sampled (Jun et al., Citation2010). Although there is also the ATP system to measure the microbial presence on surfaces, it is not a very good tool to sample biofilms, particularly when biofilms are mature as the transference of the ATP inside the biofilm is very low. In these situations, low ATP numbers would be obtained, indicating low microbial load, when in fact there might be a mature biofilm composed by a high concentration of microorganisms. Consequently, using the biodetector will help to control the hygienic state of the food-processing surfaces and making decisions in regard to cleaning and disinfection procedures within seconds, preventing cross-uncontrolled contamination from surfaces to foods.
Conclusions
Many attempts have been made to better understand the main causes of cross-contamination to food products. Food-contact surfaces are known to be the principal site of contamination and controlling them is a key to food hygiene. The results obtained in this study indicate that with the use of the solution that contained hydrogen peroxide, phosphonates, surfactans, and bleaching agents, biofilms of specific microorganisms on different surfaces can be visually detected. The data demonstrated that the strongest biofilm identified was P. aeruginosa, followed by E. coli, S. Thyphimurium, L. monocytogenes, and S. aureus. Biofilms formed by L. brevis used as a negative control were not detected when the biodetector was applied because this microorganism does not have catalase activity. Based on the results obtained and the literature survey, it can be concluded that the developed biodetector as a biofilm detection system is a potentially powerful tool to prevent both the transmission of foodborne diseases and the spoilage of food originating from food-contact surfaces.
Acknowledgments
The authors thank Dolors Busquets enormously for her technical assistance. This study was supported by Research Project grants RTA2014-00045-C03-03 (INIA) from the Spanish Ministry of Economy and Competitiveness. The authors alone are responsible for the content and writing of the paper. Authors acknowledge to Mrs. Sarah Davis for English grammar revision.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alavi, H. E. D., & Hansen, L. T. (2013). Kinetics of biofilm formation and desiccation survival of Listeria monocytogenes in single and dual species biofilms with Pseudomonas fluorescens, Serratia proteamaculans or Shewanella baltica on food-grade stainless steel surfaces. Biofouling, 29, 1253–1268.
- Annous, B. A., Fratamico, P. M., & Smith, J. L. (2009). Quorum sensing in biofilms: Why bacteria behave the way they do. Journal of Food Science, 74, 24–36.
- Anonymous. (2002). ISO-13697:2002. Chemical disinfectants and antiseptics. Quantitative non-porous surface test for the evaluation of bactericidal and/or fungicidal activity of chemical disinfectants used in food, industrial, domestic and institutional.
- Anonymous. (2007). ISO-22196:2007. Plastics - Measurement of antibacterial activity on plastics surfaces.
- Bagge-Ravn, D., Ng, Y., Hjelm, M., Christiansen, J. N., Johansen, C., & Gram, L. (2003). The microbial ecology of processing equipment in different fish industries. Analysis of the microflora during processing and following cleaning and disinfection. International Journal of Food Microbiology, 87, 239–250.
- Baumann, A. R., Martin, S. E., & Feng, H. (2009). Removal of Listeria monocytogenes biofilms from stainless steel by use of ultrasound and ozone. Journal of Food Protection, 72, 1306–1309.
- Beuchat, L. R., Kim, H., Gurtler, J. B., Lin, L. C., Ryu, J. H., & Richards, G. M. (2009). Cronobacter sakazakii in foods and factors affecting its survival, growth, and inactivation. International Journal of Food Microbiology, 136(2), 204–213.
- Branda, S. S., Vik, A., Friedman, L., & Kolter, R. (2005). Biofilms: The matrix revisited. Current Trends in Microbiology, 13, 20–26.
- Carlton, R. M., Noordman, W. H., Biswas, B., de Meester, E. D., & Loessner, M. J. (2005). Bacteriophage P100 for control of Listeria monocytogenes in foods: Genome sequence, bioinformatic analyses, oral toxicity study, and application. Regulatory Toxicology and Pharmacology, 43, 301–312.
- Chae, M. S., & Schraft, H. (2000). Comparative evaluation of adhesion and biofilm formation of different Listeria monocytogenes strains. International Journal of Food Microbiology, 62, 103–111.
- Chmielewski, R. A. N., & Frank, J. F. (2003). Biofilm formation and control in food processing facilities. Comprehensive Reviews on Food Science and Food Safety, 2, 22–32.
- Christensen, B. E., Trønnes, H. N., Vollan, K., Smidsrød, O., & Bakke, R. (1990). Biofilm removal by low concentration of hydrogen peroxide. Biofouling, 2, 165–175.
- Colagiorgi, A., Bruini, I., Di Ciccio, P. A., Zanardi, E., Ghidini, S., & Ianieri, A. (2017). Listeria monocytogenes biofilms in the wonderland of food industry. Pathogens, 6, 41–50.
- Di Ciccio, P., Vergara, A. R., Festino, A., Paludi, D., Zanardi, E., Ghidini, S., & Ianieri, A. (2015). Biofilm formation by Staphylococcus aureus on food contact surfaces: Relationship with temperature and cell surface hydrophobicity. Food Control, 50, 930–936.
- Elias, S., & Banin, E. (2012). Multi-species biofilms: Living with friendly neighbors. FEMS Microbiology Reviews, 36, 990–1004.
- Elkins, J. G., Hassett, D. J., Stewart, P. S., Schweizer, H. P., & MCDermott, T. R. (1999). Protective role of catalase in Pseudomonas aeruginosa biofilm resistance to hydrogen peroxide. Applied and Environmental Microbiology, 65(10), 4594–4600.
- Fuster-Valls, N., Hernández-Herrero, M., Marín-de-Matero, M., & Rodríguez-Jerez, J. J. (2008). Effect of different environmental conditions on the bacteria survival on stainless steel surfaces. Food Control, 19, 308–314.
- Garrett, T. R., Bhakoo, M., & Zhang, Z. (2008). Bacterial adhesion and biofilms on surfaces. Progress in Natural Science, 18(9), 1049–1056.
- Gunduz, G. T., & Tuncel, G. (2006). Biofilm formation in an ice cream plant. Antonie Van Leeuwenhoek, 89, 329–336.
- Guobjornsdottir, B., Einarsson, H., & Thorkelsson, G. (2005). Microbial adhesion to processing lines for fish fillets and cooked shrimp: Influence of stainless steel surface finish and presence of gram-negative bacteria on the attachment of Listeria monocytogenes. Food Technology and Biotechnology, 43, 55–61.
- Hannig, C., Hannig, M., Rehmer, O., Braun, G., Hellwig, E., & Al-Ahmad, A. (2007). Fluorescence microscopic visualization and quantification of initial bacterial colonization on enamel in situ. Archives Oral Biology, 52, 1048–1056.
- Holah, J. T., Betts, R. P., & Thorpe, R. H. (1989). The use of epifluorescence microscopy to determine surface hygiene. International Biodeterioration & Biodegradation, 25, 147–153.
- Hood, S., & Zottola, E. A. (1995). Biofilms in food processing. Food Control, 6, 9–18.
- Jahid, I. K., & Ha, S. (2014). The paradox of mixed-species biofilms in the context of food safety. Comprehensive Reviews of Food Science and Food Safety, 13, 990–1011.
- Jahid, I. K., Han, N., Srey, S., & Ha, S. D. (2014b). Competitive interactions inside mixed-species biofilms of Salmonella Typhimurium and cultivable indigenous microorganisms on lettuce enhance microbial resistance of their sessile cells to ultraviolet C (UV-C) irradiation. Food Research International, 55, 445–454.
- Jun, W., Kim, M. S., Cho, B. K., Millner, P. D., Chao, K., & Chan, D. E. (2010). Microbial biofilm detection on food contact surfaces by macro-scale fluorescence imaging. Journal of Food Engineering, 99, 314–322.
- Kumar, C. G., & Anand, S. (1998). Significance of microbial biofilms in food industry: A review. International Journal of Food Microbiology, 42, 9–27.
- LeChevallier, M. W., Cawthon, C. D., & Lee, R. G. (1988). Inactivation of biofilm bacteria. Applied Environmental Microbiology, 54, 2492–2499.
- Lee, K. W., Periasamy, S., Mukherjee, M., Xie, C., Kjelleberg, S., & Rice, S. A. (2014). Biofilm development and enhanced stress resistance of a model, mixed-species community biofilm. ISME Journal, 8, 894–907.
- Lee Wong, A. C. (1998). Biofilms in food processing environments. Journal of Dairy Science, 81, 2765–2770.
- Lunau, M., Lemke, A., Walther, K., Martens-Habbena, W., & Simon, M. (2005). An improved method for counting bacteria from sediments and turbid environments by epifluorescence microscopy. Environmental Microbiology, 7, 961–968.
- Manuzon, M. Y., & Wang, H. H. (2007). Mixed-species biofilms. In H. P. Blaschek, H. H. Wang, & M. E. Agle (Eds.), Biofilms in food environment (pp. 105–125). Ames, IA: Blackwell Publishing Ltd.
- Montañez-Izquierdo, V. Y., Salas-Vázquez, D. I., & Rodríguez-Jerez, J. J. (2012). Use of epifluorescence microscopy to assess the effectiveness of phage P100 in controlling Listeria monocytogenes biofilms on stainless steel surfaces. Food Control, 23, 470–477.
- Moons, P., Michiels, C. W., & Aertsen, A. (2009). Bacterial interactions in biofilms. Critical Reviews in Microbiology, 35, 157–168.
- Myszka, K., & Czaczyk, K. (2011). Bacterial biofilms on food contact surfaces - a review. Polish Journal of Food and Nutrition Sciences, 61, 173–180.
- Nivens, D. E., Co, B. M., & Franklin, M. J. (2009). Sampling and quantification of biofilms in food processing and other environments. In P. M. Fratamico, B. A. Annous, & N. W. Guenther (Eds.), Biofilms in the food and beverage industries (pp. 539–568). Abington Hall, UK: Woodhead Publishing. doi:10.1533/9781845697167.5.539
- Notermans, S., Nauta, M. J., Jansen, J., Jouve, J. L., & Mead, G. C. (1998). A risk assessment approach to evaluating food safety based on product surveillance. Food Control, 9, 217–223.
- Percival, S. L., Walker, J. T., & Hunter, P. R. (2000). Microbiological aspects on biofilms and drinking water. Boca Raton, FL: CRC Press.
- Pettipher, G. L., & Rodrigues, U. M. (1982). Rapid enumeration of microorganisms in foods by the direct epifluorescent filter technique. Applied and Environmental Microbiology, 44, 809–813.
- Phillips, C. A. (2016). Bacterial biofilms in food processing environments: A review of recent developments in chemical and bioloical control. International Journal of Food Science and Technology, 51, 1731–1743.
- Ríos-Castillo, A. G., González-Rivas, F., & Rodríguez-Jerez, J. J. (2017). Bactericidal efficacy of hydrogen peroxide-based disinfectants against gram-positive and gram-negative bacteria on stainless steel surfaces. Journal of Food Science, 82, 2351–2356.
- Sharma, M., & Anand, S. K. (2002). Biofilms evaluation as an essential component of HACCP for food/dairy processing industry – A case. Food Control, 13, 469–477.
- Shi, X., & Zhu, X. (2009). Biofilm formation and food safety in food industries. Trends in Food Science and Technology, 20, 407–413.
- Simões, M., Simões, L. C., & Vieira, M. J. (2010). A review of current and emergent biofilm control strategies. LWT Food Science and Technology, 43, 573–583.
- Stepanović, S., Cirković, I., Ranin, L., & Svabić-Vlahović, M. (2004). Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Letters in Applied Microbiology, 38, 428–432.
- Swaminathan, B., & Gerner-Smidt, P. (2007). The epidemiology of human listeriosis. Microbes and Infection, 9, 1236–1243.
- Tompkin, R. B. (2002). Control of Listeria monocytogenes in the food-processing environment. Journal of Food Protection, 65, 709–725.
- Torlak, E., & Sert, D. (2013). Combined effect of benzalkonium chloride and ultrasound against Listeria monocytogenes biofilm on plastic surface. Letters in Applied Microbiology, 57, 220–226.
- Van Houdt, R., & Michiels, C. W. (2010). Biofilm formation and the food industry, a focus on the bacterial outer surface. Journal of Applied Microbiology, 109, 1117–1131.
- Wang, H., Qi, J., Dong, Y., Li, Y., Xu, X., & Zhou, G. (2017). Characterization of attachment and biofilm formation by meat-borne Enterobacteriaceae strains associated with spoilage. LWT – Food Science and Technology, 86, 399–407.
- Whittenbury, R. (1964). Hydrogen peroxide formation and catalase activity in the lactic acid bacteria. Journal of General Microbiology, 35, 13–26.
- Yan, X., & Gurtler, J. B. (2014). Cronobacter (Enterobacter) sakazakii. In C. A. Batt & M. L. Tortorello (Eds.), Encyclopedia of food microbiology, (2nd ed. pp. 528–532). Oxford, UK: Academic Press. doi:10.1016/B978-0-12-384730-0.00382-7
- Yang, H., Kendall, P. A., Medeiros, L. C., & Sofos, J. N. (2009). Efficacy of sanitizing agents against Listeria monocytogenes biofilms on high-density polyethylene cutting board surfaces. Journal of Food Protection, 72, 990–998.
