 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The present study investigated the influence of Myrciaria dubia peel and seed extracts (PE and SE) on colour and oxidation stability of ground lamb. Instrumental colour (L*, a*, and b* values), pH, lipid oxidation (TBARS), and protein oxidation (carbonyls) were determined during 9 days of storage at 4 °C. Although the L* value was not affected by any enhancement type (BHT, PE, or SE), all samples exhibited a decrease on the a* values (P < 0.05) during refrigerated storage. M. dubia extracts increased the lipid stability in ground lamb, however no positive effect on protein oxidation was observed. PE and SE samples had lower (P < 0.05) TBARS values compared with control and BHT counterparts. Due to its antioxidant effect on lipids, peel and seed extracts of M. dubia can be considered a viable strategy to improve the oxidative stability of ground lamb during storage.
RESUMEN
El presente estudio investigó el efecto que tiene la agregación de extractos de cáscara y semillas (PE y SE) de Myrciaria dubia en el color y la estabilidad de oxidación de la carne de cordero molida. Ésta fue almacenada durante 9 días a 4 °C para determinar el color instrumental (valores de L*, a* y b*), el pH, la oxidación de lípidos (TBARS) y la oxidación de proteínas (carbonilos). Se constató que, aunque el valor de L* no fue afectado por ninguno de los tipos de mejoramiento (BHT, PE o SE), en todas las muestras se detectó una disminución de los valores de a* (P <0 05) durante el almacenamiento en refrigeración. Si bien los extractos de M. dubia aumentaron la estabilidad de lípidos en la carne de cordero molida, no se observaron efectos positivos en la oxidación de proteínas. En comparación con las contrapartes de control y BHT, las muestras de PE y SE mostraron valores TBARS (P < 0.05) más bajos. Debido a su efecto antioxidante en lípidos es posible considerar el uso de extractos de cáscara y semilla de M. dubia como una estrategia válida para mejorar la estabilidad oxidativa de la carne de cordero molida durante su almacenamiento.
1. Introduction
Meat quality deterioration involves the oxidation of both fatty acids and muscle pigments, processes which are synergistic and result in simultaneous loss of colour and odour/flavour quality (Viana, Canto, Costa-Lima, Salim, & Conte-Junior, Citation2016). In addition, proteins are also important substrates for oxidation reactions (Canto et al., Citation2016), the oxidation of myofibrillar proteins affect their functionality generally compromising the texture quality of processed meats (Cao, True, Chen, Youling, & Xiong, Citation2016).
Previous reports documented that the fat present in lamb longissimus thoracis et lumborum is composed of nearly 50% of unsaturated fatty acids where 36% are monounsaturated and 13% are polyunsaturated fatty acids (Hajji et al., Citation2016). Lipid oxidation is a well characterized three-step radical chain reaction comprised of initiation, propagation, and termination involving mainly fatty acid (R•), peroxyl (ROO•), and hydroperoxide (H2O2) radicals. These reactions yield volatile compounds in meat related to off-flavours and rancid odour (Falowo, Fayemi, & Muchenje, Citation2014). In addition, these processes are self-propagating processes and can be associated with the off-flavours and off-odours produced during meat storage (Baron & Andersen, Citation2002; Faustman, Sun, Mancini, & Suman, Citation2010; Yin & Faustman, Citation1993). Proteins are also important substrates for oxidation reactions (Canto et al., Citation2016; Lorido, Ventanas, Akcan, & Estévez, Citation2016) commonly associated with reactive oxygen species (ROS) leading to the formation for a variety of products including carbon-centred (P•), peroxyl (POO•), alkyl peroxide (POOH), and alcoxyl (PO•) radicals (Lund, Heinonen, Baron, & Estévez, Citation2011).
The food industry usually utilizes synthetic antioxidants such as butylated hydroxyanisole, butylated hydroxytoluene, tert-butylhydroquinone, and propyl gallate to mitigate the negative effects matrix oxidation of food. These synthetic antioxidants have the advantage of low cost and high efficiency (Almeida et al., Citation2015), however there is a demand-driven growing interest by the consumer market for replacement of artificial additives with compounds from natural sources (Viana, Silva, & Trindade, Citation2014). Plant tissues including bark, leaf, root, fruit, and their parts are widely studied as potential sources of phytochemicals with potential use as substitutes of synthetic molecules (Armenteros, Morcuende, Ventanas, & Estévez, Citation2016; Ganhão, Morcuende, & Estévez, Citation2010; Jia, Kong, Liu, Diao, & Xia, Citation2012). Fruit by-products are interesting raw materials due to their economical aspect, furthermore several varieties exhibit well established crops and processing chains indicating a more reliable sourcing of raw materials (Guedes-Oliveira, Salgado, Costa-Lima, Guedes-Oliveira, & Conte-Junior, Citation2016). Previous authors reported that native fruits from rainforests exhibit high contents of polyphenols and organic acids with antioxidant potential (López et al., Citation2015; Rufino et al., Citation2010).
Myrciaria dubia (camu camu) is a berry from the Amazon Rainforest known for its high content of ascorbic acid (Zanatta & Mercadante, Citation2007) with potential to be utilized as an antioxidant ingredient (Fracassetti, Costa, Moulay, & Tomás-Barberán, Citation2013). In addition, the antioxidant activity of M. dubia is not solely attributed to ascorbic acid, but also to the high levels of phenolic compounds such as flavonols, anthocyanins, and ellagic acid (Gonçalves, Lajolo, & Genovese, Citation2010; Reynertson, Yang, Jiang, Basile, & Kennelly, Citation2008; Rufino et al., Citation2010; Zanatta & Mercadante, Citation2007). Peel and seed are by products from M. dubia processing, indicating a potential source of inexpensive raw material for the isolation of bioactive compounds to be utilized in food and pharmaceutical industries (Deng et al., Citation2012). Also, these by-products exhibit higher contents of antioxidant molecules than the pulp (Deng et al., Citation2012; Fracassetti et al., Citation2013).
Although there is an increasing wealth of information about natural alternatives to synthetic antioxidants utilized to improve the colour and chemical stability of meat products (Shah, Bosco, & Mir, Citation2014), there is a lack of information about the influence of M. dubia extracts on lamb products quality parameters. Ground meat is not allowed to contain synthetic antioxidants (Tarté, Citation2009), nonetheless the present study utilized butylated hydroxytoluene and ground lamb as a model with simpler chemical composition and manufacturing procedure than other processed meats. In this context, the aim of this study was to evaluate the efficacy of the extracts obtained from M. dubia peel and seed on the mitigation of lipid and protein oxidation, and colour stability of ground lamb during refrigerated storage.
2. Materials and methods
2.1 Plant material
Myrciaria dubia (HBK) McVaugh samples were provided by the Sindicato Rural de Iguape (24° 42′ 29″ S, 47° 33′ 19″ W) (Iguape, São Paulo, Brazil). The berries were manually peeled and the seeds separated. Seed and peel of M. dubia were individually dehydrated with forced-air for 24 h at 25 °C to constant weight. Dried seeds and peels were individually ground in a burr grinder (MSS-1B, Hario, Japan), sieved through a 0.125 mm mesh, and stored in amber-glass flasks at −20 °C before extraction.
2.2 Seeds and peel extracts of M. dubia
Seed and peel extracts were individually obtained by homogenizing the ground samples in 50% (v/v) ethanol in water solvent at a solute-to-solvent ratio of 1:10 (w/v). Extraction was done on a rotary shaker (TS – 2000 A, Biomixer, China) at 60 rpm for 120 min at 25 °C. The extracts were centrifuged at 13,000 × g (Sorvall ST16R, Thermo Scientific, São Paulo, Brazil) for 5 min at 4 °C, and the supernatants were kept at 4 °C in amber-glass flasks.
2.3 Total phenolic content (TPC)
Total phenolic content of the extracts was estimated by Folin-Ciocalteu method and expressed as mg gallic acid equivalents (GAE) per ml of the extract (Ainsworth & Gillespie, Citation2007). The standard curve ranging from 0.0 to 1.2 mg GAE was plotted with an r-square of 0.991. The extracts exhibited 1.8 ± 0.1 and 2.0 ± 0.2 mg of GAE/mL for the peel and seed starting materials, respectively. Furthermore, the extraction procedure of the peel yielded 1.2 g, while the seed provided 1.7 g per 100 g of starting material.
2.4 Application of seed and peel extracts in lamb meat
2.4.1 Lamb patties and storage conditions
Eigth untrimmed longissimus thoracis et lumborum (LTL) muscle whole cuts were obtained 36 hours post-mortem from Texel animals harvested at 10 months of age; each muscle derived from a different animal. The cuts were individually ground and divided into four batters to be enhanced to 110% of the intial weight with either water (negative control; NC); 100 mg/kg BHT (positive control; PC); 100 mg/kg peel extract (PE); or 100 mg/kg seed extract (SE). Immediately after the enhancement the batches were homogenized for 5 minutes at low speed utilizing a bowl mixer equiped with a heavy dough hook attachment (SX8401, Arno, Brazil), and formed into patties (5.5 cm diameter × 1.5 cm thickness). Patties were placed on polystyrene trays with soaker pads, over-wraped with polyvinyl chloride (PVC) film (0.014 mm thickness, O2 transmition rate 320 cm3· m–2· d–1, Orleplast Ind. e Com de Plásticos Ltda, Orleans, Santa Catarina, Brazil), and stored for 9 days at 4 °C. The PVC film was not in direct contact with the sample surface.
2.4.2 Proximate composition and pH value
Moisture, lipids, protein and ash contents, and pH values were determined using official methods (AOAC, Citation2012). The pH value was determined from samples homogenized at 1:9 sample-to-water ratio utilizing a pHmeter (DM-22, Digimed, Campo Grande, Brazil).
2.4.3 Instrumental color
The instrumental colour was monitored using a spectrophotometer (CM-600D, Konica Minolta Sensing Inc., Osaka, Japan) equipped with illuminant D65, 8 mm aperture, and 10° standard observer (AMSA, Citation2012). Lightness (L* value), redness (a* value) and yellowness (b* value) parameters were recorded at three different locations on the patties surface. The colour difference between treatments was calculated considering the following equation:
2.4.4 Lipid oxidation
TBARS was reported as mg MDA/kg meat (Yin, Faustman, Riesen, & Williams, Citation1993). Briefly, 5 g of sample were homogenized with 23 mL of 11% trichloroacetic acid (TCA) utilizing an Ultra Turrax T18 basic (IKA, Wilmington, NC, USA) for two cycles consisting of 1 min of homogenization followed by 1 min of ice-cold water bath. Aliquots of 1.5 mL of the homogenate were centrifuged at 15,000 × g for 15 min at 4 ºC (Sorvall ST16R, Thermo Scientific, São Paulo, Brazil) to obtain a clear supernatant from which, 1.0 mL was reacted with an equal volume of 20 mM thiobarbituric acid solution. The test tubes were briefly vortexed and incubated for 18 h at 25 °C in the dark for colour development. Absorbance at 532 nm were recorded utilizing an UV-1800 spectrophotometer (Shimadzu Corporation, Kyoto, Japan). Concentration of TBARS was calculated from standard curve (r2 = 0.997) prepared with Malondialdehyde tetrabutylammonium salt (Sigma-Aldrich, Brazil).
2.4.5 Protein oxidation
Protein oxidation was estimated based on 2,4-dinitrophenylhydrazine (DNPH) derivatization method (Oliver, Ahn, Moerman, Goldstein, & Stadtman, Citation1987) with modifications (Armenteros, Heinonen, Ollilainen, Toldrá, & Estévez, Citation2009). Three grams of sample were homogenized utilizing Ultra Turrax T18 basic (IKA, Wilmington, NC, USA) in 30 mL of 0.75 M KCl solution (pH 7.4) for 90 s. Aliquots of 1.0 mL were centrifuged at 15,000 × g for 15 min at 4 ºC (Sorvall ST16R, Thermo Scientific, São Paulo, Brazil), and then two 100 µL aliquots were individually vortexed with 1.0 mL of 10% TCA solution and centrifuged at 5,000 × g for 5 min at 4 °C. The pellets were either dissolved in 1.0 mL of 2 N HCl or with 1.0 mL of 20 mM DNPH in 2 N HCl solution for protein and carbonyls quantification, respectively. The homogenates were incubated for 1 hour at 25 °C with intermittent brief vortexing every 15 min following precipitation with 20% TCA and centrifugation at 11,000 × g for 10 min at 4 ºC. The pellets were washed three times with 1.0 mL ethanol:ethyl acetate (1:1) to remove unbound DNPH, and finally dissolved in 1.0 mL of 20 mM sodium phosphate (pH 6.5) and 6 M guanidine hydrochloride with the aid of a water bath at 50 ºC. A final centrifugation step at 11,000 × g for 10 min at 4 ºC was utilized to remove insoluble particles. Absorbance values at 370 nm (carbonyl content) and 280 nm (protein content) were recorded and the carbonyls contents were expressed as nmol of carbonyl per mg of protein.
2.5 Statistical analysis
Eight batches (N = 8) of each treatment, from individual loins, were manufactured to evaluate the influence of enhancement (NC, PC, PE, and SE) and refrigerated storage (0, 5, and 9 days) on pH, instrumental colour, lipid oxidation and protein oxidation of ground lamb. Analysis of variance at 95% of confidence level (P < 0.05) was carried out to determine the significance of the independent factors (treatments and storage days) followed by Tukey’s test utilizing XLStat software (Addinsoft, Paris, France).
3. Results and discussion
3.1 Proximate composition and pH value
The ground lamb exhibited 66.47 ± 1.00 g/100g moisture, 18.32 ± 0.39 g/100g protein, 0.95 ± 0.02 g/100g ash, and 13.53 ± 0.14 g/100g lipid. The values of proximate composition of ground LD were similar to the ones reported in the literature for the lean LD (Fernandes, Freire, Carrer, & Trindade, Citation2013; Jaworska, Czaudema, Przybylski, & Rozbicka-Wieczorek, Citation2016). Nonetheless, in the present study the lipid content was greater than the values reported in the literature due to the formulation effect as the ground lamb contained the fat layer that was not trimmed from the LD in order to emulate a high fat content in a raw lamb meat product.
The pH for peel and seed extract presented values of 2.84 and 2.48, respectively. The pH value of ground lamb was not affected (P > 0.05) by the addition of either BHT, PE, or SE (). The pH values of lamb meat are in the range of 5.4 – 5.9 (Fregonesi et al., Citation2014; Gonçalves, Zapata, Rodrigues, & Borges, Citation2004), which encloses the initial values (day 0) in the present experiment. Other authors also reported no influence of the addition of fruit extracts (red grape) on the pH value of pork burgers (Garrido, Auqui, Martí, & Linares, Citation2011). Furthermore, the pH values increased significantly (P < 0.05) in all the treatments at day 5 and 9 refrigerated storage. The literature reported an increase on pH values of lamb during 10 days of refrigerated storage due to bacterial and endogenous enzymatic degradation of proteins yielding alkaline molecules (Muela, Sañudo, Campo, Medel, & Beltrán, Citation2010).
Table 1. Instrumental colour and pH values of ground lamb (N = 8) enhanced with Myrciaria dubia (camu camu) extracts and stored at 4 °C for 9 days.
Tabla 1. Color instrumental y valores de pH de carne de cordero molida (N = 6) mejorada con extractos de Myrciaria dubia (camu camu) y almacenada a 4 °C durante 9 días.
3.2 Instrumental colour
The addition of either peel or seed extract did not affect (P > 0.05) the L* values at the beginning of the refrigerated storage (day 0; ). However, at day 9 the patties enhanced with SE exhibited lower L* values (P < 0.05) than the other treatments. Differently than other treatments, SE patties exhibited the highest protein oxidation throughout the refrigerated storage, potentially affecting the L* values. Oxidation of meat pigments is responsible for the decrease of lightness values in meat (Villalobos-Delgado, González-Mondragón, Govea, Andrade, & Santiago-Castro, Citation2017). In addition, the decrease in lightness is potentially due to the characteristic dark colour of extracts (Selani et al., Citation2011). In the present study, PE and SE presented L* values of 26.90 and 28.70, respectively. M. dubia PE was dark-red in colour (a* 8.00; b* 2.82), while SE exhibited an orange colour (a* 2.58; b* 20.12). Beef patties enhanced with edible plants extracts (crown daisy, pumpkin, chamnamul, fatsia, leek, bok choy, acanthopanax, butterbur, soybean, and flower heads of broccoli) exhibited a decrease in L* values (Kim et al., Citation2013b).
Peel extract decreased lamb patties redness (a* values) at the beginning of the refrigerated storage (day 0; P < 0.05), whereas NC presented the highest value for redness 15.88; PE exhibited the lowest value at 11.13. Whilst at day 9, BHT-enhanced patties (PC) had the highest redness 8.06 (P < 0.05), negative control an intermediate value (7.05), and PE the lowest value at 6.51. However, neither the synthetic antioxidant (BHT in PC) nor the M. dubia extracts (PE and SE) were able to attenuate the decrease on redness. Oxidation of myoglobin into metmyoglobin negatively affects the redness values (Kim, Cho, & Han, Citation2013a; Realini, Guàrdia, Díaz, García-Regueiro, & Amau, Citation2015) therefore, it was expected that by increasing the levels of reducing chemicals it would result in decreased pigment oxidation and improved colour stability. In addition, according to Zang et al. (Citation2016) the carotenoids level of extracts may increase the red colour, however M. dubia fruits may not contain sufficient levels of carotenoids (Rufino et al., Citation2010) to affect the a* values in ground lamb.
Yellowness (b* values) was affected (P < 0.05) by the addition of seed extract, at day 0 NC showed the highest b* value (18.14) while SE the lowest (13.33). However, at days 5 and 9 the treatments with M. dubia extracts presented a more pronounced decrease (P < 0.05) in yellowness than NC and PC. At the end of storage (day 9), NC kept the highest yellowness (12.87) and SE the lowest (10.19). Previous authors reported that red grape pomace extract, decreased yellowness in pork burgers due to the phenolic compounds that act as potent free radical scavengers preventing lipid oxidation (Garrido et al., Citation2011). Radical species from lipid oxidation may also promote the oxidation of colour pigments and the formation of metmyoglobin. Metmyoglobin levels were among the most important factors contributing to meat redness and yellowness (Luciano et al., Citation2009).
The ΔE values in the , describe the colour differences between treatments. A ΔE of 1 or less is not perceptible by human eyes (Sharifzadeh, Clemmensen, Borgaard, Stoier, & Ersboll, Citation2014). All the treatments at days 0, 5 and 9 of storage presented perceptible differences of colour; whereas only NC and PC at day 5 presented ΔE value lower than 1. ΔE consists of the changes in lightness, redness and yellowness. The variation presented for PE and SE in those parameters () may explain the different values of ΔE for patties with peel and seed extracts. This behaviour can be explained by the compounds found in each extract (Fracassetti et al., Citation2013).
Table 2. Colour difference between ground lamb (N = 8) enhanced with Myrciaria dubia (camu camu) extracts and stored at 4 °C for 9 days.
Tabla 2. Diferencia en el color en las muestras de carne de cordero molida (N = 6) mejorada con extractos de Myrciaria dubia (camu camu) y almacenadas durante 9 días a 4 °C.
3.3 Lipid oxidation
Peel and seed extracts affected (P < 0.05) the lipid oxidation values (); in general, throughout the whole storage period lamb patties enhanced with PE and SE exhibited lower (P < 0.05) TBARS values than NC and PC counterparts. Similar to the present study, other authors reported a decrease on the lipid oxidation rate in ground beef enhanced with Plinia cauliflora peel and leafy green vegetable extracts (Almeida et al., Citation2015; Kim et al., Citation2013a). As expected, the refrigerated storage promoted an increase (P < 0.05) on lipid oxidation potentially due to enzymatic and non-enzymatic reactions (Grau, Guardiola, Boatella, & Codony, Citation2000; Selani et al., Citation2011).
Figure 1. Extent of lipid oxidation of patties enhanced with Myrciaria dubia (camu camu) extracts and stored at 4 °C for 9 days. Bars represent standard error.NC: negative control; PC: positive control (100 mg/kg BHT); PE: 100 mg/kg of peel extract; and SE: 100 mg/kg of seed extract. a–b: means with different letters indicate difference amongst storage period (P < 0.05). x–y: means with different letters indicate difference amongst enhancement (P < 0.05).
Figura 1. Grado de oxidación de lípidos de los medallones mejorados con extractos de Myrciaria dubia (camu camu) y almacenados durante 9 días a 4 °C. Las barras representan el error estándar. NC: control negativo; PC: control positivo (100 mg/kg de BHT); PE: 100 mg/kg de extracto de cáscara; y SE: 100 mg/kg de extracto de semilla. a–b: Las medias que figuran con distintas letras indican diferencias en el periodo de almacenamiento (P < 0.05). x–y: Las medias que figuran con distintas letras indican diferencias en los tipos de mejoramiento (P < 0.05).
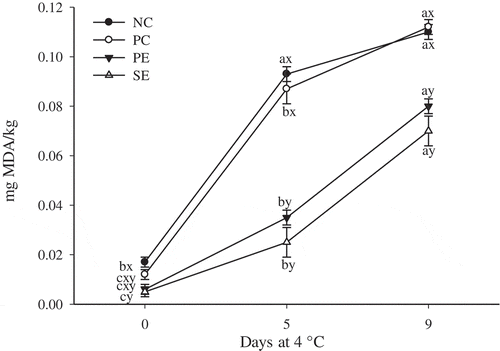
The protective effect of the M. dubia extracts on lipid oxidation may be associated with the phenolic molecules present in this fruit. Previous reports documented that M. dubia fruit contains considerable amounts of anthocyanins (42.2 mg/100g), flavonoids (20.1 mg/100g), carotenoids (0.4 mg/100g), and ascorbic acid (1,882 mg/100g), which exhibit antioxidant activity and the ability to quench free radicals (Kaneshima et al., Citation2016; Mariem et al., Citation2014; Rufino et al., Citation2010). Furthermore, anthocyanins, flavonoids, carotenoids and ascorbic acid may participate in the stabilization of lipid free radicals into less reactive forms, resulting in the protection against lipid oxidation (Jridi et al., Citation2018). Jia et al. (Citation2012) attributed the inhibitory effect of black currant extract on lipid oxidation to its phenolic compounds and other biochemical compounds that contribute to the antioxidant activity. The present study illustrates the efficiency of the peel and seed extracts elaborated from M. dubia processing on the prevention of lipid oxidation in lamb patties.
3.4 Protein oxidation
Protein oxidation in ground lamb was evaluated by the formation of protein carbonyls (). In this study, only PC provided a protection against protein oxidation represented by lower (P < 0.05) carbonyl levels than the other treatments at the end of refrigerated storage (day 9). Furthermore, the addition of SE promoted an increase (P < 0.05) on the protein carbonyls content at day 0; and from day 5 until the end of the refrigerated storage, SE, PE, and NC exhibited similar (P > 0.05) carbonyl content values. It was previously reported that seed of M. dubia has higher contents of ellagitannins than peel and pulp (Fracassetti et al., Citation2013) and different content of phenolic compounds may exhibit pro-oxidant effects in a protein-liposome system (Viljanen, Kylli, Kivikari, & Heinonen, Citation2004).
Figure 2. Extent of protein oxidation of patties enhanced with Myrciaria dubia (camu camu) extracts and stored at 4 °C for 9 days. Bars represent standard error.NC: negative control; PC: positive control (100 mg/kg BHT); PE: 100 mg/kg of peel extract; and SE: 100 mg/kg of seed extract. a–b: means with different letters indicate difference amongst storage period (P < 0.05). x–y: means with different letters indicate difference amongst enhancement (P < 0.05).
Figura 2. Grado de oxidación de proteínas en los medallones mejorados con extractos de Myrciaria dubia (camu camu) y almacenados durante 9 días a 4 °C. Las barras representan el error estándar. NC: control negativo; PC: control positivo (100 mg/kg de BHT); PE: 100 mg/kg de extracto de cáscara; y SE: 100 mg/kg de extracto de semilla. a–b: Las medias que figuran con distintas letras indican diferencias en el periodo de almacenamiento (P < 0.05). x–y: Las medias que figuran con distintas letras indican diferencias en los tipos de mejoramiento (P < 0.05).
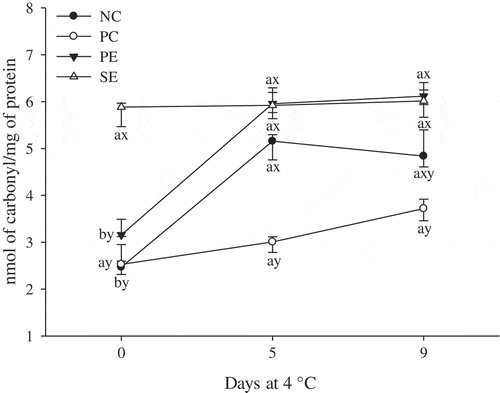
The influence of fruit extracts on protein oxidation in meat products is a controversial topic as some authors documented that extracts from strawberry tree fruit, hawthorn, dog rose, elm-leaf black-berry (Ganhão et al., Citation2010), white grape (Jongberg, Torgren, Gunvig, Skibsted, & Lund, Citation2013), and dog rose (Armenteros, Morcuende, Ventanas, & Estévez, Citation2013) exerted antioxidant activity against the formation of protein carbonyls in meat, whereas other authors documented no protective effect against protein carbonylation when cured beef and pork hams were enhanced with apple pomace (Sun, Zhang, Zhou, Xu, & Peng, Citation2010). These conflicting results may be attributed to differences on the structure and levels of phytochemicals present in fruits (Estévez, Kylli, Puolanne, Kivikari, & Heinonen, Citation2008; Jia et al., Citation2012) and the extraction procedure (Jia et al., Citation2012; Jongberg et al., Citation2013). Moreover, plant phenolics are known as redox–active molecules that present antioxidant activities depending on their concentrations and the presence of other compounds (Sabeena Farvin, Grejsen, & Jacobsen, Citation2012; Zhang, Lin, Leng, Huang, & Zhou, Citation2013). The interaction between phenolic compounds and the formation of protein carbonyls are not well known, thus further investigations are necessary to improve the understanding about such mechanisms (Jia et al., Citation2012; Zhang et al., Citation2013).
The present study demonstrated that peel and seed extracts of Myrciaria dubia protected ground lamb against lipid peroxidation, however this effect was not observed on protein oxidative stability. These results suggest that the replacement of artificial antioxidants by M. dubia peel and seed extracts can be considered a viable solution in order to improve the lipid oxidative stability during the storage of lamb with minimal negative effect on the colour parameters. Further studies are necessary to improve the processing yield, and to identify and isolate the active phytochemicals from M. dubia by-products in order to optimize the application of extracts and promote the development of lamb products.
Acknowledgements
The authors thank the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (process no. E-26/201.185/2014, FAPERJ, Brazil) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (process no. 311,361/2013-7, CNPq, Brazil). The authors also thank the Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil) for providing the scholarship to Juliana M.G. Oliveira, and the Sindicato Rural de Iguape and Cordeiro Rei for providing the lamb samples.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- A.O.A.C. (2012). Official methods of Analysis (19th ed.). Maryland: Association of Official Analytical Chemists.
- Ainsworth, E. A., & Gillespie, K. M. (2007). Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nature Protocols, 2, 875—877.
- Almeida, P. L., Lima, S. N., Costa, L. L., Oliveira, C. C., Damasceno, K. A., Santos, B. A., & Campagnol, P. C. B. (2015). Effect of jabuticaba peel extract on lipid oxidation, microbial stability and sensory properties of Bologna-type sausages during refrigerated storage. Meat Science, 110, 9—14.
- AMSA. (2012). Meat color measurement guidelines (pp. 100). Champaign, IL, USA: American Meat Science Association.
- Armenteros, M., Heinonen, M., Ollilainen, V., Toldrá, F., & Estévez, M. (2009). Analysis of protein carbonyls in meat products by using the DNPH method, fluorescence spectroscopy and liquid chromatography-electrospray ionization-mass spectrometry (LC–ESI–MS). Meat Science, 83, 104—112.
- Armenteros, M., Morcuende, D., Ventanas, J., & Estévez, M. (2016). The application of natural antioxidants via brine injection protects Iberian cooked hams against lipid and protein oxidation. Meat Science, 116, 253—259.
- Armenteros, M., Morcuende, D., Ventanas, S., & Estévez, M. (2013). Application of natural antioxidants from strawberry tree (Arbutus unedo L.) and dog rose (Rosa canina L.) to frankfurters subjected to refrigerated storage. Journal of Integrative Agriculture, 12, 1972—1981.
- Baron, C. P., & Andersen, H. J. (2002). Myoglobin-induced lipid oxidation, a review. Journal of Agricultural and Food Chemistry, 50, 3887—3897.
- Canto, A. C. V. C. S., Costa-Lima, B. R. C., Suman, S. P., Monteiro, M. L. G., Viana, F. M., Salim, A. P. A. A., … Conte-Junior, C. A. (2016). Color attributes and oxidative stability of longissimus lumborum and psoas major muscles from Nellore bulls. Meat Science, 121, 19—26.
- Cao, Y., True, A. D., Chen, J., Youling, L., & Xiong, Y. L. (2016). Dual role (Anti- and Pro-oxidant) of gallic acid in mediating myofibrillar protein gelation and gel in vitro digestion. Journal of Agricultural and Food Chemistry, 64, 3054—3061.
- Deng, G.-F., Shen, C., Xu, X.-R., Kuang, R.-D., Guo, Y.-J., Zeng, L.-S., … Li, H.-B. (2012). Potential of fruit wastes as natural resources of bioactive compounds. International Journal of Molecular Sciences, 13, 8308—8323.
- Estévez, M., Kylli, P., Puolanne, E., Kivikari, R., & Heinonen, M. (2008). Oxidation of skeletal muscle myofibrillar proteins in oil-in-water emulsions: Interaction with lipids and effect of selected phenolic compounds. Journal of Agricultural and Food Chemistry, 56, 10933—10940.
- Falowo, A. B., Fayemi, P. O., & Muchenje, V. (2014). Natural antioxidants against lipid-protein oxidative deterioration in meat and meat products: A review. Food Research International, 64, 171—181.
- Faustman, C., Sun, Q., Mancini, R., & Suman, S. P. (2010). Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Science, 86, 86—94.
- Fernandes, R. P. P., Freire, M. T. A., Carrer, C. C., & Trindade, M. A. (2013). Evaluation of physicochemical, microbiological and sensory stability of frozen stored vacuum-packed lamb meat. Journal of Integrative Agriculture, 12, 1946—1952.
- Fracassetti, D., Costa, C., Moulay, L., & Tomás-Barberán, F. A. (2013). Ellagic acid derivatives, ellagitannins, proanthocyanidins and other phenolics, vitamin C and antioxidant capacity of two powder products from camu-camu fruit (Myrciaria dubia). Food Chemistry, 139, 996—1002.
- Fregonesi, R. P., Portes, R. G., Aguiar, A. M. M., Figueira, L. C., Gonçalves, C. B., Arthur, V., … Trindade, M. A. (2014). Irradiated vacuum-packed lamb meat stored under refrigeration: Microbiology, physicochemical stability and sensory acceptance. Meat Science, 97, 151—155.
- Ganhão, R., Morcuende, D., & Estévez, M. (2010). Protein oxidation in emulsified cooked Burger patties with added fruit extracts: Influence on colour and texture deterioration during chill storage. Meat Science, 85, 402—409.
- Garrido, M. D., Auqui, M., Martí, N., & Linares, M. B. (2011). Effect of two different red grape pomace extracts obtained under different extraction systems on meat quality of pork burgers. LWT - Food Science and Technology, 44, 2238—2243.
- Gonçalves, A. E. S. S., Lajolo, F. M., & Genovese, M. I. (2010). Chemical composition and antioxidant/antidiabetic potential of Brazilian native fruits and commercial frozen pulps. Journal of Agricultural and Food Chemistry, 58, 4666—4674.
- Gonçalves, L. A. G., Zapata, J. F. F., Rodrigues, M. C. P., & Borges, A. S. (2004). Efeitos do sexo e do tempo de maturação sobre a qualidade da carne ovina. Ciência e. Tecnologia de Alimentos, 24, 459—467.
- Grau, A., Guardiola, F., Boatella, J., & Codony, R. (2000). Measurement of 2-thiobarbituric acid values in dark chicken meat through derivative spectrophotometry: Influence of various parameters. Journal of Agricultural and Food Chemistry, 48, 1155—1159.
- Guedes-Oliveira, J. M., Salgado, R. L., Costa-Lima, B. R. C., Guedes-Oliveira, J., & Conte-Junior, C. A. (2016). Washed cashew apple fiber (Anacardium occidentale L.) as fat replacer in chicken patties. LWT - Food Science and Technology, 71, 268—273.
- Hajji, H., Joy, M., Ripoll, G., Smeti, S., Mekki, I., Molino Gahete, F., … Atti, N. (2016). Meat physicochemical properties, fatty acid profile, lipid oxidation and sensory characteristics from three North African lamb breeds, as influenced by concentrate or pasture finishing diets. Journal of Food Composition and Analysis, 48, 102—110.
- Jaworska, D., Czaudema, M., Przybylski, W., & Rozbicka-Wieczorek, A. J. (2016). Sensory quality and chemical composition of meat from lambs fed diets enriched with fish and rapeseed oils, carnosic acid and seleno-compounds. Meat Science, 119, 185—192.
- Jia, N., Kong, B., Liu, Q., Diao, X., & Xia, X. (2012). Antioxidant activity of black currant (Ribes nigrum L.) extract and its inhibitory effect on lipid and protein oxidation of pork patties during chilled storage. Meat Science, 91, 533—539.
- Jongberg, S., Torgren, M. A., Gunvig, A., Skibsted, L. H., & Lund, M. N. (2013). Effect of green tea or Rosemary extract on protein oxidation in Bologna type sausages prepared from oxidatively stressed pork. Meat Science, 93, 538—546.
- Jridi, M., Mora, L., Souissi, N., Aristoy, M.-C., Nasri, M., & Toldrá, F. (2018). Effect of active gelatin coated with henna (L. inermis) extract on beef meat quality during chilled storage. Food Control, 84, 238—245.
- Kaneshima, T., Myoda, T., Nakata, M., Fujimori, T., Toeda, K., & Nishizawa, M. (2016). Antioxidant activity of C-Glycosidic ellagitannins from the seeds and peel of camu-camu (Myrciaria dubia). LWT - Food Science and Technology, 69, 76—81.
- Kim, S.-J., Cho, A. R., & Han, J. (2013a). Antioxidant and antimicrobial activities of leafy green vegetable extracts and their applications to meat product preservation. Food Control, 29, 112—120.
- Kim, S.-J., Min, S. C., Shin, H.-J., Lee, Y.-J., Cho, A. R., Kim, S. Y., & Han, J. (2013b). Evaluation of the antioxidant activities and nutritional properties of ten edible plant extracts and their application to fresh ground beef. Meat Science, 93, 715—722.
- López, C. C., Mazzarrino, G., Rodríguez, A., Fernández-López, J., Pérez-Álvarez, J. A., & Viuda-Martos, M. (2015). Assessment of antioxidant and antibacterial potential of borojo fruit (Borojoa patinoi Cuatrecasas) from the rainforests of South America. Industrial Crops and Products, 63, 79—86.
- Lorido, L., Ventanas, S., Akcan, T., & Estévez, M. (2016). Effect of protein oxidation on the impaired quality of dry-cured loins produced from frozen pork meat. Food Chemistry, 196, 1310—1314.
- Luciano, G., Monahan, F. J., Vasta, V., Pennisi, P., Bella, M., & Priolo, A. (2009). Lipid and colour stability of meat from lambs fed fresh herbage or concentrate. Meat Science, 82, 193—199.
- Lund, M. N., Heinonen, M., Baron, C. P., & Estévez, M. (2011). Protein oxidation in muscle foods: A review. Molecular Nutrition & Food Research, 55, 83—95.
- Mariem, C., Sameh, M., Nadhem, S., Soumaya, Z., Najiba, Z., & Raoudha, E. G. (2014). Antioxidant and antimicrobial properties of the extracts from Nitraria retusa fruits and their applications to meat product preservation.
- Muela, E., Sañudo, C., Campo, M. M., Medel, I., & Beltrán, J. A. (2010). Effect of freezing method and frozen storage duration on instrumental quality of lamb throughout display. Meat Science, 84, 662—669.
- Oliver, C. N., Ahn, B. W., Moerman, E. J., Goldstein, S., & Stadtman, E. R. (1987). Aged-elated changes in oxidized proteins. Journal of Biological Chemistry, 262, 5488—491.
- Realini, C. E., Guàrdia, M. D., Díaz, I., García-Regueiro, J. A., & Amau, J. (2015). Effects of acerola fruit extract on sensory and shelf-life of salted beef patties from grinds differing in fatty acid composition. Meat Science, 99, 18—24.
- Reynertson, K. A., Yang, H., Jiang, B., Basile, M. J., & Kennelly, E. J. (2008). Quantitative analysis of antiradical phenolic constituents from fourteen edible Myrtaceae fruits. Food Chemistry, 109, 883—890.
- Rufino, M. S. M., Alves, R. E., Brito, E. S., Pérez-Jiménez, J., Saura-Calixto, F., & Mancini-Filho, J. (2010). Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chemistry, 121, 996—1002.
- Sabeena Farvin, K. H., Grejsen, H. D., & Jacobsen, C. (2012). Potato peel extract as a natural antioxidant in chilled storage of minced horse mackerel (Trachurus trachurus): Effect on lipid and protein oxidation. Food Chemistry, 131, 843—851.
- Selani, M. M., Contreras-Castillo, C. J., Shirahigue, L. D., Gallo, C. R., Plata-Oviedo, M., & Montes-Villanueva, N. D. (2011). Wine industry residues extracts as natural antioxidants in raw and cooked chicken meat during frozen storage. Meat Science, 83, 397—403.
- Shah, M. A., Bosco, S. J. D., & Mir, S. A. (2014). Plant extracts as natural antioxidants in meat and meat products. Meat Science, 98, 21—33.
- Sharifzadeh, S., Clemmensen, L. H., Borgaard, C., Stoier, S., & Ersboll, B. K. (2014). Supervised feature selection for linear and non-linear regression of L* a* b* color from multispectral images of meat. Engineering Applications of Artificial Intelligence, 27, 211—227.
- Sun, W. Q., Zhang, Y. J., Zhou, G. H., Xu, X. L., & Peng, Z. Q. (2010). Effect of apple polyphenol on oxidative stability of sliced cooked cured beef and pork hams during chilled storage. Journal of Muscle Foods, 21, 722—737.
- Tarté, R. (2009). Ingredients in meat products: Properties, functionality and applications. New York, NY: Springer Link.
- Viana, F. M., Canto, A. C. V. C. S., Costa-Lima, B. R. C., Salim, A. P. A. A., & Conte-Junior, C. A. (2016). Color stability and lipid oxidation of broiler breast meat from animals raised on organic versus non-organic production systems. Poultry Science, 96, 747—753.
- Viana, M. M., Silva, V. L. S., & Trindade, M. A. (2014). Consumers’ perception of beef burgers with different healthy attributes. LWT - Food Science and Technology, 59, 1227—1232.
- Viljanen, K., Kylli, P., Kivikari, R., & Heinonen, M. (2004). Inhibition of protein and lipid oxidation in liposomes by berry phenolics. Journal of Agricultural and Food Chemistry, 52, 7419—7424.
- Villalobos-Delgado, L. H., González-Mondragón, E. G., Govea, A. Y. S., Andrade, J. R., & Santiago-Castro, J. T. (2017). Potential application of epazote (Chenopodium ambrosioides L.) as natural antioxidant in raw ground pork. LWT - Food Science and Technology, 84, 306—313.
- Yin, M. C., & Faustman, C. (1993). Influence of temperature, pH, and phospholipid composition upon the stability of myoglobin and phospholipid: A liposome model. Journal of Agricultural and Food Chemistry, 41, 853—857.
- Yin, M. C., Faustman, C., Riesen, J. W., & Williams, S. N. (1993). α-Tocopherol and ascorbate delay oxymyoglobin and phospholipid oxidation in vitro. Journal of Food Science, 58, 1273–1276.
- Zanatta, C. F., & Mercadante, A. Z. (2007). Carotenoid composition from the Brazilian tropical fruit camu-camu (Myrciaria dubia). Food Chemistry, 101, 1526—1532.
- Zhang, H., Wu, J., & Guo, X. (2016). Effects of antimicrobial and antioxidant activities of spice extracts on raw chicken meat quality. Food Science and Human Wellness, 5, 19—48.
- Zhang, L., Lin, Y. H., Leng, X. J., Huang, M., & Zhou, G. H. (2013). Effect of sage (Salvia officinalis) on the oxidative stability of Chinese-style sausage during refrigerated storage. Meat Science, 95, 145—150.
