ABSTRACT
Silver nanoparticles (Ag-NPs) were synthesized in an eco-friendly manner using aqueous Houttuynia cordata leaf extract (HLE). HLE mediated Ag-NPs (Ag-NPs/HLE) exhibited surface plasmon resonance with a maximum absorbance at 450 nm. The mean diameter of Ag-NPs was 23 nm, and their crystalline structure was confirmed via X-ray diffraction analysis. Fourier-transform infrared spectroscopy indicated that hydroxyl, carboxyl, and ketone groups of sugar or polyphenolics were involved in Ag-NPs synthesis. The contribution of reducing sugars to the reduction of Ag ions was found to be greater than that of polyphenols. NO production in LPS stimulated RAW 264.7 macrophages was more effectively inhibited by Ag-NPs/HLE than by chemically synthesized Ag-NPs. The production of the major inflammatory mediators, PGE2 and TNF-α was decreased by 73% and 66%, respectively, upon the addition of 10 μg/mL Ag-NPs/HLE. Ag-NPs biosynthesized using HLE showed improved anti-inflammatory activity compared to conventional Ag-NPs owing to the incorporation of phytochemicals.
RESUMEN
En este estudio se sintetizaron nanopartículas de plata (Ag-NPs) de una forma amigable con el medio ambiente, empleando para ello el extracto acuoso de la hoja de Houttuynia cordata (HLE). Las Ag-NPs mediadas por HLE (Ag-NPs/HLE) presentaron resonancia plasmónica de superficie con una absorción máxima de 450 nm. Se constató que el diámetro medio de las Ag-NP fue de 23 nm, confirmándose su estructura cristalina mediante el análisis de difracción por rayos-X. A través de una espectroscopia infrarroja transformada de Fourier se comprobó que los grupos de azúcar hidroxilo, carboxilo y cetona o polifenólicos intervinieron en la síntesis de las Ag-NPs. Asimismo, se comprobó que la contribución de los azúcares reductores a la disminución de los iones de Ag es mayor que la de los polifenoles. La producción de NO en los macrófagos 264.7 RAW estimulados por LPS [lipopolisacáridos] fue inhibida de manera más eficaz por las Ag-NPs/HLE que por las Ag-NPs de síntesis química. Cuando se adicionaron 10 μg/mL de Ag-NPs/HLE, la producción de los principales intermediarios inflamatorios, PGE2 yTNF-α, disminuyó en 73% y 66%, respectivamente. Las Ag-NPs biosintetizadas utilizando HLE muestran mayor actividad antiinflamatoria en comparación con las Ag-NPs convencionales, debido a la incorporación de fitoquímicos.
Introduction
Nanoparticles of nobel metals such as gold and silver have unique catalytic, optical, and biological properties that depend on their size and shape. Consequently, opportunities for the application of metallic nanoparticles in biomedical science and clinical translation are continuously emerging (Arvizo et al., Citation2012). The synthesis of metal nanoparticles by chemical or physical means poses health and environmental risks while the green synthesis of metallic nanoparticles using plant extracts provides significant advantages in safety, cost, and operation procedures Rajan, Chandran, Harper, Yun, and Kalaichelvan (Citation2015). In terms of practical applications, silver nanoparticles (Ag-NPs) synthesized from plant extracts showed antimicrobial properties against Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus subtilis, and Escherichia coli and the implementation of Ag-NPs to food packaging has been suggested (Mohanta, Panda, Bastia, & Mohanta, Citation2017; Soshnikova et al., Citation2017). Additionally, new potential application of green Ag-NPs from plant-mediated synthesis as a biopesticide has been demonstrated (Suresh et al., Citation2018).
Although effect of Ag-NPs consumption on human body varies depending on dosages and duration orally consumed commercial Ag-NPs (10 nm Ag-NPs:100 ug/day, and 33 nm Ag-NPs: 480 ug/day) for 14 days did not cause any toxicity or inflammatory problems in healthy subjects (Munger et al., Citation2014). Nayak, Ashe, Rauta, Kumari, and Nayak (Citation2016) reported that Ag-NPs synthesized from Azadirachta indica bark extract significantly suppressed the proliferation of MG-63 osteosarcoma cancer cells and ultimately led to apoptosis by chromatin condensation.
Houttuynia cordata (H. cordata) Thunb., a herb that is widely distributed in Asia, has been used as a traditional medicine for the alleviation of microbial infections and swelling (Tian, Zhao, Guo, & Yang, Citation2011). Previous studies have indicated that H. cordata leaf extract (HLE) suppresses murine salmonellosis (Kim et al., Citation2008) and exhibits anti-obesity effects in high-fat diet-induced obese mice (Miyata, Koyama, & Yazawa, Citation2010).
Inflammation is a self-defense and repairing response by the body against tissue injury or pathogenic microorganisms. However, inadequate regulation of inflammatory responses leads to chronic inflammatory disorders such as obesity, atherosclerosis, and cancer (García-Lafuente, Guillamón, Villares, Rostagno, & Martínez, Citation2009). Lee, Ahn, Kim, Lee, and Kim (Citation2015) reported that HLE (70% ethanol) significantly inhibited inflammatory biomarkers such as NO and IL-6 in human lung epithelial cells and the oral administration of HLE significantly suppressed lung inflammatory response in acute lung injury mice. Furthermore, Li, Zhou, Zhang, and He (Citation2011) reported that the selective inhibition of cyclooxygenase-2 (COX-2) is one of the main mechanisms for the anti-inflammatory activity of HLE.
In this study, Ag-NPs were biosynthesized using aqueous HLE. This study aims to characterize the biosynthesized Ag-NPs and to identify the major contributors to their effects on the synthesis and stabilization of Ag-NPs. In addition, the anti-inflammatory effects of biosynthesized Ag-NPs on lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages were assessed.
Materials and methods
Materials
Dried H. cordata leaves were purchased from local herbal market in Daegu, Korea. RAW 264. 7 cells were obtained from ATCC (Manassas, VA, USA). Dulbecco’s Modified Eagle’s Medium (DMEM), penicillin-streptomycin solution and fetal bovine serum (FBS) were purchased from WelGENE Inc. (Daegu, Korea). Prostaglandin E2 (PGE2) assay kits were obtained from Cayman Chemical Company (Ann Arbor, MI, USA) and tumor necrosis factor (TNF-α) enzyme-linked immunosorbent assay kits were purchased from eBioscience (San Diego, CA, USA). AgNO3 was purchased from Daejung Chem. (Seoul, Korea). Polyvinylpyrrolidone (PVP)-functionalized silver nanospheres (20 nm) and other chemical reagents were obtained from Sigma-Aldrich Inc. (St. Louis, MO, USA)
Preparation of HLE
H. cordata leaves were carefully washed with distilled water and grounded after drying at 37°C. The resulting leaf powder (30 g) was extracted with distilled water (600 mL) by boiling for 2 h. The aqueous extract was then filtered through a Whatman no.1 filter paper and lyophilized.
Total phenol, total flavonoid, and reducing sugar contents of HLE
Total phenol and flavonoid contents of the HLE were determined by the method of Xu, Yuan, and Chang (Citation2007). Total phenol content was expressed as milligram gallic acid equivalent (GAE) per gram HLE, and total flavonoid content was expressed as milligram catechin equivalent (CTE) per gram HLE. Dinitrosalicylic acid colorimetric assay (Gusakov, Kondratyeva, & Sinitsyn, Citation2011) was used to determine reducing the sugar content of HLE.
Synthesis of Ag-NPs
The HLE was redissolved in distilled water (5 mg/mL) and used to synthesize Ag-NPs on the same day. The reconstituted HLE was mixed with an AgNO3 solution (1 mM) at a ratio of 5:95 (v/v) and placed in a 25°C water bath for 7 h. When the color of the reaction mixture had changed to red-brown, the solution was centrifuged at 12,000 rpm for 1 h to separate Ag-NPs. The pellets were collected after washing three times with distilled water. The recovered Ag-NPs were lyophilized and used for further experiments. For comparison purposes, Ag-NPs of different sizes (10, 20, and 30 nm) were chemically synthesized by the method of Agnihotri, Mukherji, and Mukherji (Citation2014) using NaBH4 and trisodium citrate. The average size of the chemically synthesized Ag-NPs was confirmed by transmission electron microscopy (TEM).
Characterization of Ag-NPs
The kinetics of Ag-NPs synthesis were observed using a UV-vis spectrophotometer (Ultra spec 2100 pro, Amersham Life Sciences, Piscataway, NJ, USA). Ag-NPs were characterized via Fourier-transform infrared (FT-IR), X-ray diffraction (XRD), and TEM. The FT-IR spectra of HLE and Ag-NPs were obtained from KBr pellets using a Vertex 70 FT-IR unit (Bruker, Germany) in a reflectance mode at a resolution of 4 cm−1. The diffraction pattern of the Ag-NPs was obtained using an Ultima IV X-ray diffractometer (Rigajku, Japan) at a voltage of 40 kV and a current of 40 mA with CuKα1 radiation (λ = 1.5406 Å). The appearance, size, and shape of the Ag-NPs were observed by TEM (JEM-2100F, JEOL Ltd, Japan). TEM samples were prepared by drop-coating the Ag-NPs solution into formvar-coated copper grids (200 mesh, Ted Pella Inc., Redding, CA, USA).
Cell culture
RAW 264.7 macrophages were cultured as previously described (Lee & Imm, Citation2017). After 24 h from cell seeding, the cells were further incubated for 24 h in the presence of samples and LPS (0.5 μg/mL). The culture supernatants were collected for NO, TNF-α, and PGE2 analysis.
Cell viability
The cytotoxicity of the samples was evaluated using MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5 diphenyltetrazolium bormide] assay. After pre-incubation of the cells with samples for 24 h, MTT solution (0.8 μL/mL) was added. The MTT solution was discarded in 3 h, and the optical density (OD at 540 nm) of formazan crystals solubilized in DMSO was determined using a plate reader (Biotek, Winooski, VT, USA). The cell viability was calculated by comparing the OD of the sample with that of the control.
Nitric oxide (NO)
Nitric oxide production was determined based on the Griess reaction. In brief, the culture medium (100 μL) was mixed with the equal volume of Griess reagent and the absorbance at 546 nm was measured using a plate reader.
TNF-α and PGE2
The concentrations of TNF-α and PGE2 in the culture medium were quantified using enzyme-linked immunosorbent assay (ELISA) assay kits according to the manufacturer’s instructions.
Statistical analysis
All quantitative determinations were done in triplicate, and data were expressed as mean ± standard deviation (SD). Statistical analysis was performed via one-way analysis of variance (ANOVA) using the SPSS statistical software (SPSS Inc., IL, Chicago, USA). Significant differences of between treatments (p < 0.05) were determined using the Duncan’s multiple comparison test.
Results and discussion
Synthesis and characterization of HLE-mediated Ag-NPs (Ag-NPs/HLE)
UV-vis and TEM analysis
The synthesis of Ag-NPs/HLE was monitored by UV-vis spectroscopy. The color of AgNO3 solution gradually turned into brownish-red upon addition of HLE [)]. Unique nanoparticle colors are expressed by surface plasmon resonance (SPR), which occurs owing to the collective oscillation of nanoparticle electrons with a periodic change in the electromagnetic field. This SPR absorption is dependent on the particle size, shape, stage of aggregation, and the dielectric constant of the solvent (Jain, Huang, El-Sayed, & El-Sayed, Citation2007). The peak wavelength of the Ag-NPs/HLE was 446 nm, and the absorbance intensity increased as the reduction of silver ion proceeded. The maximum absorption of the SPR band was maintained in the range of 446 ~ 450 nm for 7 h with no sign of band broadening.
Figure 1. UV-vis spectra (A), TEM image (B), size frequency distribution (C), and SAED pattern (D) of Ag-NPs synthesized using HLE.
HLE: aqueous Houttuynia cordata leaf extract, SAED: selected area electron diffraction. Changes in the UV-vis absorption spectra as a function of time were monitored for 7 h at 25°C.
Figura 1. Espectro UV-vis (A), imagen TEM (B), distribución de frecuencia de tamaños (C) y patrón SAED (D) de las Ag-NPs sintetizadas usando HLE.
HLE: extracto acuoso de hojas de Houttuynia cordata, SAED: difracción electrónica de área selecta. Se monitorearon los cambios en el espectro de absorción UV-vis en función del tiempo durante 7 h a 25°C.
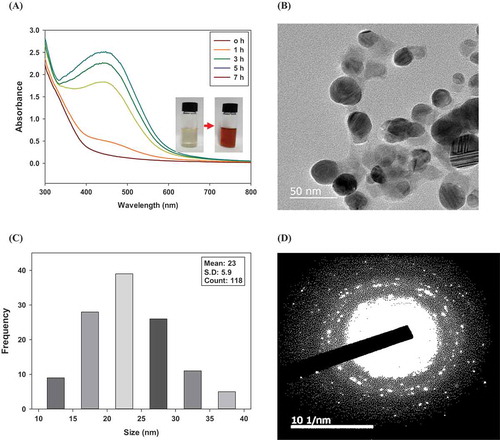
Considering that peak shift is an indication of a size change, the Ag-NPs/HLE are highly stable at room temperature (25°C). In addition, no difference in SPR peak was detected for up to 14 days during the refrigerated storage of Ag-NPs (data not shown). Mock, Barbic, Smith, Schultz, and Schultz (Citation2002) examined the effect of particle shape on the SPR of Ag-NPs. They reported that the shape of the Ag-NPs changes from spherical (near 450 nm) to pentagonal (near 530 nm) to triangular (670 nm) as the peak SPR wavelength increases. Khan et al. (Citation2013) reported that concentration of plant extract and reaction temperature play an important role in obtaining the shape and size distribution of the synthesized nanoparticles as these parameters affect the rate of reduction and stabilization. Bankar, Joshi, Kumar, and Zinjarde (Citation2010) investigated the effect of pH in reaction mixture during banana peel extract-mediated Ag-NPs synthesis. They reported that the intensity and shade of brown colors varied depending on pH (3.5–5.0). No color change was observed at pH 2.0 while precipitation occurred at higher than pH 5. This might be due to the pH-dependent behavior of functional groups involved in the reduction of silver ions.
The microscopic structure and particle size distribution of the Ag-NPs/HLE were investigated using TEM. TEM images of the Ag-NPs/HLE confirmed that the Ag-NPs are spherical particles with diameters in the range of 10 to 40 nm and have an average diameter of 23 nm. [,)]. The selected area electron diffraction (SAED) pattern of the Ag-NPs/HLE comprises concentric rings with intermittent dots, indicating a face-center-cubic crystal structure [)].
X-ray diffraction (XRD)
Four distinct diffraction peaks with 2θ values of 38.12°, 44.32°, 64.5°, and 77.42° appeared in the XRD analysis of the Ag-NPs/HLE (). These peaks represent the 111, 200, 220, and 311 planes, respectively, of face-centered cubic (fcc) geometry of Ag-NPs. A comparison of the data obtained with those from International Center Diffraction Data (ICDD) powder diffraction card NO. 03–065-2871 confirmed the formation of metallic silver. Thus, the crystalline nature of Ag-NPs indicated by the SAED pattern was confirmed by XRD. Dauthal and Mukhopadhyay (Citation2016) reported that metallic nanoparticles synthesized from plant resources facilitate spherical shape formation and they growth along with crystal planes since reduction to metalic nanoparticles from metal ions is a controlled equilibrium process.
Figure 2. XRD patterns of Ag-NPs synthesized using HLE.
HLE: aqueous Houttuynia cordata leaf extract.
Figura 2. Patrones XRD de las Ag-NPs sintetizadas utilizando HLE.
HLE: extracto acuoso de hojas de Houttuynia cordata.
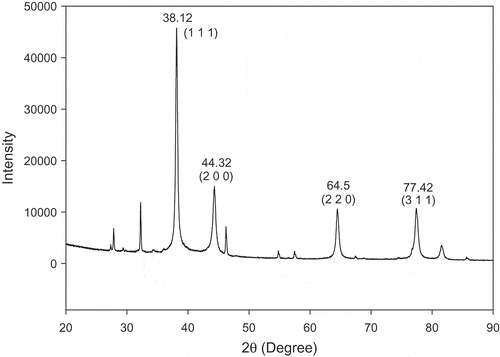
The Scherrer equation was adopted for the determination of the mean crystal size of the Ag-NPs: D = Kα/βcosθ
where D is the crystalline particle size, K is the Scherrer’s constant (0.9), α is the wavelength of the X-rays (1.54 Å), β is the peak angular width (0.006679 rad), and θ is the diffraction angle (19.06°). The calculated average particle size of the Ag-NPs was 21 nm. The estimated mean size of AgNPs/HLE showed good agreement with that obtained by TEM (23 nm).
FT-IR analysis
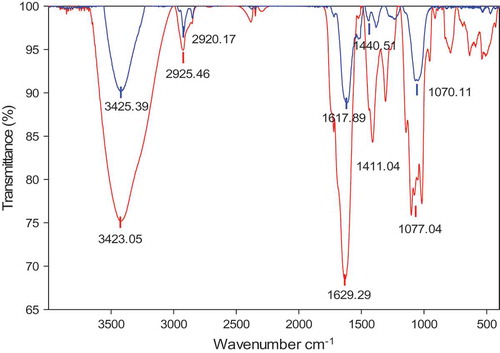
The functional groups and possible biomolecules involved in the synthesis of HLE-mediated Ag-NPs synthesis were investigated by FT-IR analysis. As shown in , the FT-IR peak patterns for HLE and Ag-NPs/HLE were well matched and only small changes were observed in the position of the absorption peaks. The intense characteristic peak at 3423–3412 cm−1 indicates the hydroxyl groups in polyphenols or carbohydrates. The peak located at approximately 2925–2920 cm−1 corresponds to C-H bond stretching vibration, while the peaks at 1629–1617 cm−1 and 1440–1411 cm−1 might be assigned to either COOH or C = O from the polyphenols or carbohydrates. The alkoxy C-O stretching vibration is found at 1077–1070 cm−1. FT-IR spectrum implies that HLE contains compounds possessing hydroxyl, carboxyl, and ketone group of sugar or polyphenolics, and they contributed to the reduction of silver ions and Ag-NPs stabilization. Moreover, the spectrum is extremely similar to that of the acidic polysaccharide fraction obtained by aqueous extraction H. cordata leaf (Tian et al., Citation2011).
Figure 3. FT-IR spectra of HLE (red) and Ag-NPs synthesized from HLE (blue).
HLE: aqueous Houttuynia cordata leaf extract.
Figura 3. Espectro FT-IR de HLE (rojo) y Ag-NPs sintetizada de HLE (azul).
HLE: extracto acuoso de hojas de Houttuynia cordata.
It has been reported that hydroxyl and carbonyl groups are actively involved in the biosynthesis of Ag-NPs using an aqueous extract of gum olibanum (Kora, Sashidhar, & Arunachalam, Citation2012). Dwivedi and Gopal (Citation2010) reported that organic acids such as oxalic acid, which are normally present in plant leaf extracts, act not only as reducing agents but also as a ligand for complexation of silver ions.
Phytoconstituents of HLE
HLE contains diverse biomolecules including flavonoids (quercetin, hyperoside, rutin, and quercitrin) (Tian et al., Citation2012) and polysaccharides dominantly composed of galacturonic acid and galactose (Tian et al., Citation2011). These compounds have redox potentials and possibly act as reducing and stabilization agents. The phytochemical contents possibly responsible for Ag-NPs synthesis were determined. For the comparison purposes, HLE prepared using 50% ethanol (EtOH-HLE) was also analyzed.
As shown in , HLE-EtOH showed total polyphenol and flavonoid contents more than 2 times greater than those of aqueous HLE. However, aqueous HLE was more effective than EtOH-HLE in the synthesis of Ag-NPs. Ag-NPs were not readily produced by EtOH-HLE, no color change was observed (data not shown). This suggests that more powerful reducing agents are present in aqueous HLE than in EtOH-HLE, and the larger amount of reducing sugars in HLE might be a reason for its effectiveness in Ag-NPs synthesis.
Various phytochemicals including phenolics, flavonoids, reducing sugars, polysaccharides (starch), and organic acids (ascorbic and citric acid) are responsible for the reduction of silver ions and the stabilization of Ag-NPs (Ghosh et al., Citation2012; Kahrilas et al., Citation2014), but their relative contributions to Ag-NPs synthesis are still unclear. Low molecular weight reducing sugars most likely have better accessibility and affinity to silver ions compared to those of high molecular weight polysaccharides. Zahran, Ahmed, and El-Rafie (Citation2014) reported that stable Ag-NPs were effectively produced using alkali hydrolyzed pectin as a reducing agent. When the same concentration of soluble starch, waxy corn starch, or sucrose was used for the synthesis of Ag-NPs, the rate of reduction was dependent on the size and molecular weight of the reduction matrix and sucrose afforded the highest reduction rate (Valodkar, Bhadoria, Pohnerkar, Mohan, & Thakore, Citation2010).
Table 1. Phytochemical constituents of aqueous and 50% ethanol HLE.
Tabla 1. Componentes fitoquímicos de HLE acuoso y de 50% etanol.
Anti-inflammatory activities Ag-NPs/HLE
Cytotoxicity of Ag-NPs/HLE
The cytotoxicity of Ag-NPs/HLE was evaluated using an MTT assay. The cytotoxicity of Ag-NPs/HLE was compared with that of commercial Ag-NPs prepared with PVP (Ag-NPs/PVP). In the case of HLE itself, cytotoxicity was not observed up to 200 μg/mL in RAW 264.7 macrophages. No cytotoxic effects were found up to 10 μg/mL for all Ag-NPs, while cell viability was decreased by 20% using a 20 μg/mL of Ag-NPs/HLE treatment ().
Figure 4. Effect of silver nanoparticles on the viability of LPS-stimulated RAW 264.7 macrophages.
HLE: aqueous Houttuynia cordata leaf extract. Commercial Ag-NPs prepared using PVP were used for comparison.
Figura 4. Efecto de las nanopartículas de plata en la viabilidad de los macrófagos 264.7 RAW estimulados por LPS.
HLE: extracto acuoso de hojas de Houttuynia cordata. Para fines de comparación se utilizaron las Ag-NPs comerciales preparadas usando PVP.
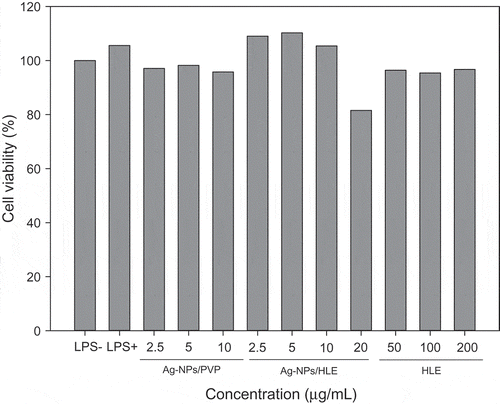
Macrophages are considered as the first cells in the body to exhibit toxicity since they internalize nanoparticles via endocytosis. Pratsinis, Hervella, Leroux, Pratsinis, and Sotiriou (Citation2013) suggested two Ag-NPs-induced toxicity mechanisms for macrophages. They proposed that small Ag-NPs (<10 nm) cause toxicity mainly by releasing Ag+ ions to the cytoplasm, whereas large Ag-NPs (>10 nm) induce toxicity by direct interaction with the macrophages. Kaur and Tikoo (Citation2013) reported that the cytotoxicity of Ag-NPs varied depending on the types of cells and reducing agents. Raw 264.7 macrophages presented less toxicity than skin and lung epithelial cells. Ag-NPs synthesized using NaBH4 did not show cytotoxicity up to 100 μg/mL, while Ag-NPs produced by tannic acid resulted in decreased cell viability at levels greater than 25 μg/mL. In the current study, based on the results of cell viability, the anti-inflammatory effects of Ag-NPs/HLE were evaluated at 2.5, 5, and 10 μg/mL.
Anti-inflammatory effects of Ag-NPs/HLE
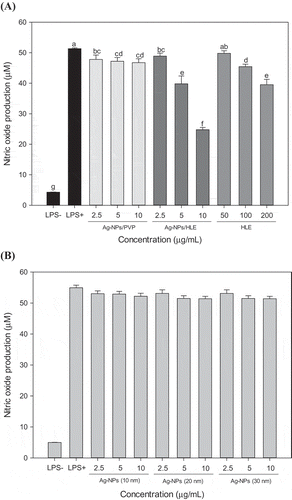
The effects of Ag-NPs/HLE treatment on the production of NO, PGE2, and TNF-α in RAW 264.7 macrophages were determined as an index of anti-inflammatory activity. NO plays an important role in physiological and pathological processes in the body including immune responses. Excessive NO production by inducible nitric oxide synthase (iNOS) promotes chronic inflammatory diseases such as asthma and leads to tissue damage (Coleman, Citation2001). As shown in (a), the effect of commercial Ag-NPs/PVP on NO production was marginal. NO production was decreased by only ~10% at 10 μg/mL compared to that of the control. The effect of Ag-NPs/PVP on NO production did not show significant difference regardless of their sizes [(b)]. Treatment with Ag-NPs/HLE resulted in a greater inhibitory effect on NO production than that with Ag-NPs/PVP, and NO production was decreased by 50% at 10 μg/mL. In the case of HLE, NO production was significantly decreased at levels above 50 μg/mL. Similar to this result, Park et al. (Citation2005) reported that aqueous HLE at 62.5 ~ 125 μg/mL significantly decreased NO and TNF-α productions in RAW 264.7 macrophages without causing cytotoxicity.
Figure 5. Effect of HLE-mediated (A) or chemically synthesized (B) Ag-NPs on NO production in LPS-stimulated RAW 264.7 macrophages.
HLE: aqueous Houttuynia cordata leaf extract. Silver nanoparticles of different sizes (10, 20, and 30 nm) were chemically synthesized using NaBH4 and trisodium citrate for comparison. Results are expressed as means ± SD of triplicate measurements. Bars with different letters indicate a significant difference at p < 0.05.
Figura 5. Efecto de las Ag-NPs mediadas por HLE (A) o de síntesis química (B) en la producción de NO en macrófagos 264.7 RAW estimulados por LPS.
HLE: extracto acuoso de hojas de Houttuynia cordata. De manera química se sintetizaron nanopartículas de plata de distintos tamaños (10, 20 y 30 nm), empleando para ello NaBH4 y citrato de sodio para fines de comparación. Los resultados se expresan como medias ± DE de mediciones por triplicado. Las barras con distintas letras indican que existe una diferencia significativa a p < 0.05.
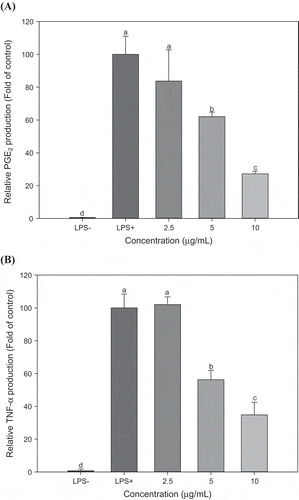
The effects of Ag-NPs/HLE on the production of the major inflammatory mediators, PGE2 and TNF-α were examined. PGE2 and TNF-α productions were significantly increased in response to LPS stimulation, but their levels were decreased by 73% and 66%, respectively, upon addition of 10 μg/mL Ag-NPs/HLE (). The two inducible enzyme isoforms, iNOS and cyclooxygenase 2 (COX-2), are expressed by the pro-inflammatory stimulation such as LPS. The synthesis of PGE2 from arachidonic acid is catalyzed by COX-2 (Coleman, Citation2001). Cross-talk between iNOS and COX-2 has been suggested, and iNOS inhibitors effectively suppressed inflammation by dual inhibition of NO and PGE2 (Salvemini et al., Citation1995).
Figure 6. Effect of Ag-NPs synthesized from HLE on productions of PGE2 (A) and TNF-α (B) in LPS-stimulated RAW 264.7 macrophages.
HLE: aqueous Houttuynia cordata leaf extract. Results are expressed as means ± SD of triplicate measurements. Bars with different letters indicate a significant difference at p < 0.05.
Figura 6. Efecto de Ag-NPs sintetizadas de HLE en la producción de PGE2 (A) y TNF-α (B) en macrófagos 264.7 RAW estimulados por LPS.
HLE: extracto acuoso de hojas de Houttuynia cordata. Los resultados se expresan como medias ± DE de mediciones por triplicado. Las barras con distintas letras indican que existe una diferencia significativa a p < 0.05.
TNF-α is a key stimulator of pro-inflammatory responses since it activates nuclear factor-kB (NF-κB), which induces expression of inflammatory genes (Sethi, Sung, & Aggarwal, Citation2008). Thus, inhibition of TNF-α production is a critical step in inflammatory signaling cascades. At this point, the anti-inflammatory mechanism of Ag-NPs/HLE is unclear but it is possible that it modulates inflammation signaling such as that mediated by NF-κB and mitogen-activated protein kinase pathways.
David et al. (Citation2014) demonstrated that Ag-NPs synthesized with black elderberry fruit extract displayed anti-inflammatory effects in HaCaT cells and in an acute inflammation rat model. The administration of Ag-NPs decreased pro-inflammatory cytokines production and had a protective effect against UV-B exposure. Banerjee and Narendhirakannan (Citation2011) reported that Ag-NPs synthesized from Syzygium cumini seed extract showed greater antioxidant activity than that of the seed extract. However, possible mechanisms for the improved antioxidant activity of Ag-NPs have not been suggested. The preferential adsorption of polyphenols on the surface of nanoparticles and their increased surface area might provide greater opportunities for interaction with RAW 264. 7 cells. This result also suggests that the biological activities of biosynthesized Ag-NPs can be improved incorporating phytochemicals.
Conclusion
Stable anti-inflammatory Ag-NPs having a mean diameter of 23 nm were synthesized using HLE in an eco-friendly manner. The contribution of reducing sugars was greater than that of polyphenols in the reduction of Ag ions and the subsequent stabilization of the Ag-NPs. NO production was more effectively inhibited in LPS-stimulated RAW 264.7 macrophages by Ag-NPs/HLE compared to that by differently sized chemically synthesized Ag-NPs. The productions of inflammatory mediators, including TNF-α and PGE2, were significantly decreased by Ag-NPs/HLE. Thus, the improvement of Ag-NP bioactivity imparted by incorporation of phytochemicals can be used to broaden their applications in biomedical fields.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Agnihotri, S., Mukherji, S., & Mukherji, S. (2014). Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Advances, 4(8), 3974–3983.
- Arvizo, R. R., Bhattacharyya, S., Kudgus, R. A., Giri, K., Bhattacharya, R., & Mukherjee, P. (2012). Intrinsic therapeutic applications of noble metal nanoparticles: Past, present and future. Chemical Society Reviews, 41(7), 2943–2970.
- Banerjee, J., & Narendhirakannan, R. T. (2011). Biosynthesis of silver nanoparticles from Syzygium cumini (L.) seed extract and evaluation of their in vitro antioxidant activities. Digest Journal of Nanomaterials and Biostructures, 6(3), 961–968.
- Bankar, A., Joshi, B., Kumar, A. R., & Zinjarde, S. (2010). Banana peel extract mediated novel route for the synthesis of silver nanoparticles. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 368(1), 58–63.
- Coleman, J. W. (2001). Nitric oxide in immunity and inflammation. International Immunopharmacology, 1(8), 1397–1406.
- Dauthal, P., & Mukhopadhyay, M. (2016). Noble metal nanoparticles: Plant-mediated synthesis, mechanistic aspects of synthesis, and applications. Industrial and Engineering Chemistry Research, 55, 9557–9577.
- David, L., Moldovan, B., Vulcu, A., Olenic, L., Perde-Schrepler, M., Fischer-Fodor, E., … Filip, G. A. (2014). Green synthesis, characterization and anti-inflammatory activity of silver nanoparticles using European black elderberry fruits extract. Colloids and Surfaces B: Biointerfaces, 122, 767–777.
- Dwivedi, A. D., & Gopal, K. (2010). Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 369(1), 27–33.
- García-Lafuente, A., Guillamón, E., Villares, A., Rostagno, M. A., & Martínez, J. A. (2009). Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflammation Research, 58(9), 537–552.
- Ghosh, S., Patil, S., Ahire, M., Kitture, R., Kale, S., Pardesi, K., & Chopade, B. A. (2012). Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. International Journal of Nanomedicine, 7, 483.
- Gusakov, A. V., Kondratyeva, E. G., & Sinitsyn, A. P. (2011). Comparison of two methods for assaying reducing sugars in the determination of carbohydrase activities. International Journal of Analytical Chemistry, 2011. Article ID 283658.
- Jain, P. K., Huang, X., El-Sayed, I. H., & El-Sayed, M. A. (2007). Review of some interesting surface plasmon resonance-enhanced properties of noble metal nanoparticles and their applications to biosystems. Plasmonics, 2(3), 107–118.
- Kahrilas, G. A., Haggren, W., Read, R. L., Wally, L. M., Fredrick, S. J., Hiskey, M., & Owens, J. E. (2014). Investigation of antibacterial activity by silver nanoparticles prepared by microwave-assisted green syntheses with soluble starch, dextrose, and arabinose. ACS Sustainable Chemistry & Engineering, 2(4), 590–598.
- Kaur, J., & Tikoo, K. (2013). Evaluating cell specific cytotoxicity of differentially charged silver nanoparticles. Food and Chemical Toxicology, 51, 1–14.
- Khan, M., Khan, M., Adil, S. F., Tahir, M. N., Tremel, W., Alkhathlan, H. Z., & Siddiqui, M. R. H. (2013). Green synthesis of silver nanoparticles mediated by Pulicaria glutinosa extract. International Journal of Nanomedicine, 8, 1507.
- Kim, G. S., Kim, D. H., Lim, J. J., Lee, J. J., Han, D. Y., Lee, W. M., … Kim, S. (2008). Biological and antibacterial activities of the natural herb Houttuynia cordata water extract against the intracellular bacterial pathogen salmonella within the RAW 264.7 macrophage. Biological and Pharmaceutical Bulletin, 31(11), 2012–2017.
- Kora, A. J., Sashidhar, R. B., & Arunachalam, J. (2012). Aqueous extract of gum olibanum (Boswellia serrata): A reductant and stabilizer for the biosynthesis of antibacterial silver nanoparticles. Process Biochemistry, 47(10), 1516–1520.
- Lee, D., & Imm, J. Y. (2017). AMP kinase activation and inhibition of nuclear factor‐kappa B (NF‐κB) translocation contribute to the anti‐inflammatory effect of tricin. Journal of Food Biochemistry, 41(2), e12293.
- Lee, J. H., Ahn, J., Kim, J. W., Lee, S. G., & Kim, H. P. (2015). Flavonoids from the aerial parts of Houttuynia cordata attenuate lung inflammation in mice. Archives of Pharmacal Research, 38(7), 1304–1311.
- Li, W., Zhou, P., Zhang, Y., & He, L. (2011). Houttuynia cordata, a novel and selective COX-2 inhibitor with anti-inflammatory activity. Journal of Ethnopharmacology, 133(2), 922–927.
- Miyata, M., Koyama, T., & Yazawa, K. (2010). Water extract of Houttuynia cordata Thunb. leaves exerts anti-obesity effects by inhibiting fatty acid and glycerol absorption. Journal of Nutritional Science and Vitaminology, 56(2), 150–156.
- Mock, J. J., Barbic, M., Smith, D. R., Schultz, D. A., & Schultz, S. (2002). Shape effects in plasmon resonance of individual colloidal silver nanoparticles. The Journal of Chemical Physics, 116(15), 6755–6759.
- Mohanta, Y. K., Panda, S. K., Bastia, A. K., & Mohanta, T. K. (2017). Biosynthesis of silver nanoparticles from Protium serratum and investigation of their potential impacts on food safety and control. Frontiers in Microbiology, 8(article), 626.
- Munger, M. A., Radwanski, P., Hadlock, G. C., Stoddard, G., Shaaban, A., Falconer, J., & Deering-Rice, C. E. (2014). In vivo human time-exposure study of orally dosed commercial silver nanoparticles. Nanomedicine and Nanotechnology, 10, 1–9.
- Nayak, D., Ashe, S., Rauta, P. R., Kumari, M., & Nayak, B. (2016). Bark extract mediated green synthesis of silver nanoparticles: Evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Materials Science and Engineering, C 58, 44–52.
- Park, E., Kum, S., Wang, C., Park, S. Y., Kim, B. S., & Schuller-Levis, G. (2005). Anti-inflammatory activity of herbal medicines: Inhibition of nitric oxide production and tumor necrosis factor-α secretion in an activated macrophage-like cell line. American Journal of Chinese Medicine, 33(3), 415–424.
- Pratsinis, A., Hervella, P., Leroux, J. C., Pratsinis, S. E., & Sotiriou, G. A. (2013). Toxicity of silver nanoparticles in macrophages. Small, 9(15), 2576–2584.
- Rajan, R., Chandran, K., Harper, S. L., Yun, S. I., & Kalaichelvan, P. T. (2015). Plant extract synthesized silver nanoparticles: An ongoing source of novel biocompatible materials. Industrial Crops and Products, 70, 356–373.
- Salvemini, D., Settle, S., Masferrer, J., Seibert, K., Currie, M., & Needleman, P. (1995). Regulation of prostaglandin production by nitric oxide; an in vivo analysis. British Journal of Pharmacology, 114(6), 1171–1178.
- Sethi, G., Sung, B., & Aggarwal, B. B. (2008). TNF: A master switch for inflammation to cancer. Frontiers in Bioscience, 13(2), 5094–5107.
- Soshnikova, V., Kim, Y. J., Singh, P., Huo, Y., Markus, J., Ahn, S., … Yang, D. C. (2017). Cardamom fruits as a green resource for facile synthesis of gold and silver nanoparticles and their biological applications. Artificial cells. Nanomedicine and Biotechnology. doi:10.1080/21691401.2017.1296849
- Suresh, U., Murugan, K., Panneerselvam, C., Rajaganesh, R., Roni, M., Aziz, A. T., … Benelli, G. (2018). Suaeda maritima-based herbal coils and green nanoparticles as potential biopesticides against the dengue vector Aedes aegypti and the tobacco cutworm Spodoptera litura. Physiological and Molecular Plant Pathology, 101, 225–235.
- Tian, L., Shi, X., Yu, L., Zhu, J., Ma, R., & Yang, X. (2012). Chemical composition and hepatoprotective effects of polyphenol-rich extract from Houttuynia cordata tea. Journal of Agricultural and Food Chemistry, 60(18), 4641–4648.
- Tian, L., Zhao, Y., Guo, C., & Yang, X. (2011). A comparative study on the antioxidant activities of an acidic polysaccharide and various solvent extracts derived from herbal Houttuynia cordata. Carbohydrate Polymers, 83(2), 537–544.
- Valodkar, M., Bhadoria, A., Pohnerkar, J., Mohan, M., & Thakore, S. (2010). Morphology and antibacterial activity of carbohydrate-stabilized silver nanoparticles. Carbohydrate Research, 345(12), 1767–1773.
- Xu, B. J., Yuan, S. H., & Chang, S. K. C. (2007). Comparative analyses of phenolic composition, antioxidant capacity, and color of cool season legumes and other selected food legumes. Journal of Food Science, 72, 2.
- Zahran, M. K., Ahmed, H. B., & El-Rafie, M. H. (2014). Facile size-regulated synthesis of silver nanoparticles using pectin. Carbohydrate Polymers, 111, 971–978.
