?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Tree peony seed oil (TPSO) is novel edible oil enriched in omega-3 polyunsaturated fatty acid. To inhibit TPSO oxidation, several individual and combined natural antioxidants were added and evaluated by peroxide value, DPPH and ABTS radical scavenging capacity under accelerated storage condition. The effects were also compared with synthetic antioxidants. We found that all the natural compounds have certain antioxidant effect at different concentrations, especially tea polyphenols (TP) that significantly prevented TPSO from oxidation at the concentration of 0.04%. Additionally, composite antioxidants significantly improved antioxidant effects, especially synergist ascorbyl palmitate (AP) was added. The composite 0.02% TP + 0.01% DMY (dihydromyricetin) + 0.01% AP exhibited the most effective inhibition effect on TPSO oxidation and strongest DPPH and ABTS radical scavenging capacity. These results demonstrated that the candidates individual and combined natural antioxidants are beneficial for lipid stabilization and health promotion, which are desirable substitutes for synthetic antioxidants.
RESUMEN
El aceite de semillas de peonías (TPSO) es un aceite comestible innovador, rico en ácido graso poliinsaturado omega-3. Para inhibir la oxidación del TPSO, se agregaron varios antioxidantes naturales individuales y combinados, evaluándose su efecto mediante su índice de peróxido y su capacidad para eliminar los radicales DPPH y ABTS bajo condiciones aceleradas de almacenaje. Los efectos de estos antioxidantes también se compararon con los efectos producidos por antioxidantes sintéticos. Al respecto se comprobó que todos los compuestos naturales tienen cierto efecto antioxidante en distintas concentraciones, en particular los polifenoles de té (TP) a una concentración de 0.04%; claramente, éstos evitaron la oxidación del TPSO. Asimismo, los antioxidantes compuestos mejoraron significativamente los efectos antioxidantes, sobre todo con la adición del sinergista palmitato ascorbilo (AP). El compuesto 0.02% TP + 0.01% DMY (dihidromiricetina) + 0.01% AP mostró el efecto inhibidor de la oxidación de TPSO más eficaz y la mayor capacidad de eliminación de los radicales DPPH y ABTS. Estos resultados dan cuenta de que las opciones de antioxidantes naturales individuales y combinados son benéficas para la estabilización de los lípidos y la promoción de la salud, lo que los hace sustitutos favorables de los antioxidantes sintéticos.
1. Introduction
Paeonia ostii ‘Feng Dan’ is well known for its ornamental and medical values, which is indigenous to China and has a long history of artificial cultivation (Cheng, Li, & Yu, Citation1998; Li et al., Citation2009; Picerno et al., Citation2011). Recently, a series of study showed that oil extracted from seed of tree peony contains abundant unsaturated fat acids (UFAs, >90%), especially α-linolenic acid (ALA, >40%) (Li et al., Citation2015a, Citation2015b), which makes it more easily absorbed and plays an important role in fighting against different kinds of diseases such as cancer, cardiovascular, inflammatory and autoimmune diseases (Kim, Nam, Kim, Hayes, & Lee, Citation2014; Kolanowski, Citation2008; Shahidi, Citation2015; Simopoulos, Citation2006). However, it is a severe problem that becomes tree peony seed oil vulnerable to oxidation owing to its high content of UFAs and less internal antioxidants.
Lipid oxidation consists of auto-oxidation, photo-oxidation, enzymatic oxidation and ketonic oxidation, while auto-oxidation is the most common reaction during storage and processing of edible oils (Taghvaei & Jafari, Citation2015). There are many factors that affect lipid oxidation, such as degree of unsaturation, temperature, oxygen, light, moisture, metal ions, and antioxidants (Choe & Min, Citation2006; Frankel, Citation2005; Upadhyay & Mishra, Citation2016; Zuta, Simpson, Zhao, & Leclerc, Citation2007). Thereinto, the level of unsaturation is the most crucial factor. Lipid oxidation not only reduces nutritional quality, flavor and taste of food, but also produces something harmful on human health causing aging, heart diseases, emphysema, mutagenesis, and carcinogenesis by generating free radicals (Bera, Lahiri, & Nag, Citation2006; Guo et al., Citation2016; Rehman & Salariya, Citation2006). Therefore, it is essential to take measures to inhibit oxidation for this novel edible oil. Currently, the addition of antioxidants was chosen as the preferred measures for controlling the oxidation of lipids.
Antioxidants can prevent free radical-induced cell and biological target damage by preventing the formation of radicals, scavenging them or by promoting their decomposition (Young & Woodside, Citation2001). At present, there were mainly two kinds of antioxidants to prevent lipid oxidation, namely synthetic antioxidants and natural antioxidants. Synthetic antioxidants such as tertbutyl hydroquinone (TBHQ), butylated hydroxyl toluene (BHT), butylated hydroxyl anisole (BHA) and propyl gallate (PG) have a strong antioxidant capacity. However, considerable researches have shown that synthetic antioxidants may have potential toxicity that promotes DNA damages (Dolatabadi & Kashanian, Citation2010; Hou, Citation2003; Shahidi & Zhong, Citation2010). Due to health concern from consumers, considerable researches focus on natural antioxidants (Kathirvel & Rupasinghe, Citation2011). Natural antioxidants mostly come from spices and herbs, such as green tea, apple peel, olive leaves, rosemary and bamboo leaves, which are known to contain many bioactive phytochemicals that can slow down lipid oxidation (Bubonja-Sonje et al., Citation2011; Bouaziz, Fki, Jemai, Ayadi, & Sayadi, Citation2008; Sekhon-Loodu, Warnakulasuriya, Rupasinghe, & Shahidi, Citation2013; Shahidi & Zhong, Citation2010; Taghvaei & Jafari, Citation2015; Ye, Wang, Duncan, Eigel, & O’Keefe, Citation2015; Zhang et al., Citation2008). Compared with synthetic antioxidants, natural antioxidants are safer and more thermally stable (Chang, Ostric-Matijasevic, Hsieh, & Huang, Citation2010). In addition, natural antioxidants add to the nutraceutical value of edible oil, which have properties of cardioprotection, antibacteria, anticancer, etc. (Knekt et al., Citation2002; Shahidi & Zhong, Citation2010; Wojcik, Burzynska-Pedziwiatr, & Wozniak, Citation2010).
The internal antioxidant contents of different oil are diverse. Therefore, the interaction of external antioxidants and internal antioxidants can lead to different preservation effects on different edible oil. Although many natural antioxidants have been widely reported in many edible oils, the reports were mainly focused on individual natural antioxidant prevention effects on lipid oxidation (Bera et al., Citation2006; Chen et al., Citation2014; Guo et al., Citation2016; Martínez, Penci, & Ixtaina et al., Citation2013). Nevertheless, few researches were associated with the effects of concentration and synergism. In the present study, we aimed to evaluate the concentration, synergistic and radical scavenging effects of several potential novel antioxidants, namely bamboo leaf (AOB), tea polyphenols (TP), rosemary extract (RE), paeonol (PAE), dihydromyricetin (DMY), vitamin E (VE), phytic acid (PA) and propolis (PR), under accelerated storage condition for inhibiting oxidation of TPSO. Additionally, a comparison was made between plant-based antioxidant and synthetic antioxidant to provide evidences for replacement of synthetic antioxidant.
2. Materials and methods
2.1. Chemicals and reagents
Antioxidants of bamboo leaf (AOB), tea polyphenols (TP, 98%), rosemary extract (RE, 60% carnosic acid), paeonol (PAE, ≧98%), dihydromyricetin (DMY, 98%), vitamin E (VE, 50%), phytic acid (PA, 70%), propolis (PR), citric acid (CA), ascorbyl palmitate (AP, ≧95%), 3(2)-tert-Butyl-4-hydroxyanisole (BHA, 99%), 2.6-ditertiary butyl-p-cresol (BHT, 98%), tertiary butylhydroquinone (TBHQ, 98%), and propyl gallate (PG, 98%) were purchased from Yuan Ye Bio-Technology Co., Ltd (Shanghai, China). 1,1-Diphenyl-2-picrylhydrazyl (DPPH), (±)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) and 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) were obtained from Sigma–Aldrich (Germany). All antioxidants were food grade, while other chemicals and reagents were analytical grade.
2.2. Materials
Seeds of Paeonia ostii ‘Feng Dan’ (moisture 5.10%, kernel rate 67.51%) were obtained from Yangling Jinshan Agricultural Science and Technology Limited Company. The seeds were plump and had uniform size. After peeling, crushing and screening, tree peony seed powders were obtained. Then, crude peony seed oil was collected through pressing by QYZ 500 automatic hydraulic press under the condition of 80°C and 35 MPa for 5 h. Finally, the refined peony seed oil was produced through hydration degumming, alkali deacidifiction, and decoloring processes (Lee, Jung, & Yoon, Citation2014).
2.3. Analysis of peroxide value (PV)
The PV of TPSO samples were measured according to the previous report with some modification (Chen et al., Citation2014). Two gram TPSO samples were dissolved in 30 mL of chloroform-glacial acetic acid (2:3, v/v) blended solution. Then, 1 mL saturated solution of KI was added. The mixture was shaken by incubator shaker (KYC 100B, Shanghai Fuma Test Equipment Co., LTD, China) for 0.5 min and was then kept in dark for 5 min. After the addition of 75 mL distilled water, the mixture was titrated against sodium thiosulfate (0.002 M) until the yellow color almost disappeared. Then, about 0.5 mL of starch indicator (10 g/L) solution was added. Titration was continued until the blue color just disappeared. Blank was also determined under similar conditions. Peroxide value (meq/kg) was calculated according to the formula:
c is the concentration of sodium thiosulphate (M); V2 and V1 are the volumes of sodium thiosulfate exhausted by samples and blank, respectively (mL), m is the mass of TPSO (g).
2.4. Antioxidants base liquid preparation
RX, TBHQ, and VE were directly dissolved into tree peony seed oil (TPSO) to get a concentration of 1% (w/w), the same concentration of other antioxidants solution was dissolved in ethanol (95%). Each base liquid of antioxidant was added to TPSO at different concentrations, respectively, according to the legal limit of China and used ultrasound to promote dissolution in oil. A control sample was prepared with the same oil without any antioxidant. All the samples were kept at 4°C before use.
2.5. Relative DPPH radical scavenging capacity
DPPH radical scavenging capacity was determined by using the method of Chiari and Li with some modifications (Chiari et al., Citation2014; Li, Lin, Gao, Han, & Chen, Citation2012). Briefly, 0.1 mL sample dissolved in 95% ethanol were mixed with 3.9 mL DPPH (0.2 mM). The mixture was kept in dark at room temperature for 45 min, and then the absorbance was measured with UV spectrophotometer (Unico 3802, Shanghai, China) at 517 nm. The blank control solution was absolute ethanol. The negative control solution was prepared by mixing 3.9 mL of 0.2 mM DPPH solution with 0.1 mL of absolute ethanol. To confirm the reproducibility of the data, the experiments were repeated three times. A curve of absorption value versus concentration of Trolox was plotted. The result was calculated and expressed as micromoles of Trolox equivalents (TE) per 100 g of oil weight (µmol TE/100 g DW).
2.6. Relative ABTS radical scavenging capacity
The ABTS radical scavenging ability was measured as described as previous studies, but with some modifications (Chiari et al., Citation2014; Li et al., Citation2012). The ABTS•+ was produced by adding 10 mL ABTS diammonium salt (7.4 mM) to 10 mL potassium persulfate (2.6 mM) and kept in the dark at room temperature for 12 h. Then, the mixture was diluted with 95% ethanol to give an absorption value of 0.70 ± 0.02 at 734 nm. To determine the scavenging activity, 0.9 mL ABTS•+ reagent was mixed with 0.1 mL sample ethanolic solutions for 6 min. The blank control solution was absolute ethanol. The negative control solution was prepared by mixing 3.9 mL of 0.2 mM DPPH solution with 0.1 mL of absolute ethanol. The absorbance was measured and read on UV spectrophotometer at 734 nm. Micromoles of Trolox equivalents (TE) per 100 g of oil weight (µmol TE/100 g DW) was calculated by the standard curve of Trolox., The experiments were repeated three times to confirm the reproducibility of the data.
2.7. Lipid oxidation under accelerated storage condition
Each antioxidant has a maximum added amount according to standards for use of food additives in China. Among them, VE can be added to oil according to production needs. The inhibitive ability of antioxidants to oxidative deterioration of oil were measured by Schaal oven test. One or a mixture of natural antioxidants was added to TPSO. Then, the oil was filled into sealed bottles at 60°C for 7 days. Meanwhile, samples were shaken every day. All samples were characterized for peroxide value (POV).
2.8. Statistical analysis
All measurements were taken in triplicates, and the results were expressed as mean ± standard deviation (SD). Statistical analysis was conducted on the results obtained using one-way analysis of variance (ANOVA) analysis and Duncan’s multiple range test using the SPSS 19 software (SPSS Inc., USA). Significant levels were based on the confidence level of 95% (p < 0.05).
3. Results and discussion
3.1. Oxidation stability of tree peony seed oil
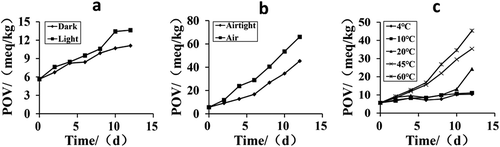
In the initial stage of lipid oxidation reaction, the generation rate of hydrogen peroxide is higher than the degradation rate, later the trend is on the contrary (Taghvaei & Jafari, Citation2015). Therefore, peroxide value can be considered as an important index reflected the degree of oxidation in the initial stage. To determine the oxidation stability of TPSO, the effects of light, air, and temperature were considered on PV.
As shown in , peroxide values showed an increasing trend along with time. Under the condition of room temperature and airtight, the PV of light-exposed TPSO samples increased relatively faster than those samples in the dark (). Compared to the air-exposed oil samples, the PV of airtight samples increased slower in the dark at 60°C condition (). It revealed that the PV of TPSO increased slowly below 20°C, while there were sharp increasing trends over 20°C and higher the temperature was, the faster the PV increased (). These results indicated that the factors of light, air, and temperature were contributors to lipid oxidation, which coincided with previous studies (Gharby et al., Citation2011; Upadhyay & Mishra, Citation2016). Edible oil standard of PV is no more than 15 meq/kg. From , we can conclude that TPSO was more prone to oxidation than common edible vegetable oil under common storage condition. Therefore, protection of TPSO from possible oxidative degradation, either by controlling storage conditions or incorporation of antioxidants in the oil, is essential to extend its shelf-life.
3.2. Effect of different natural antioxidants on oxidation stability of TPSO
3.2.1. Optimization of additional level of natural antioxidants
Each antioxidant has a limited added amount according to standards for use of food additives in most countries (Farag, Badei, & El Baroty, Citation1989). Previous studies showed that different concentrations of antioxidants showed different activities to suppress lipid oxidation (Guo et al., Citation2016). To better dissect the concentration effects of potential novel natural antioxidants for protection of lipid from oxidation, various concentrations of AOB, TP, RE, PAE, DMY, VE, PA, and PR were added into TPSO system as shown in . Accelerated oxidation test was employed and conducted according to Schaal oven method. All samples were stored in an oven at 60°C for a week and characterized by PV.
Figure 2. Effects of different concentrations of natural antioxidants on PV of TPSO. CK represent TPSO without antioxidant. Column and error bar represent mean values and standard deviation, respectively (n = 3). a-f Different superscript letters denote significant differences (p < 0.05). a: Antioxidants of bamboo leaf (AOB); b: rosemary extract (RE); c: tea polyphenols (TP); d: dihydromyricetin (DMY); e: phytic acid (PA); f: paeonol (PAE); g: propolis (PR); h: vitamin E (VE).
Figura 2. Efectos de diferentes concentraciones de antioxidantes en el PV del TPSO. CK representa el TPSO sin antioxidante. Las barras de columna y de error representan valores medios y la desviación estándar, respectivamente (n = 3). a-f Las distintas letras en superíndice indican diferencias significativas (p < 0.05). a: Antioxidantes de hojas de bambú (AOB); b: extracto de romero (RE); c: polifenoles de té (TP); d: dihidromiricetina (DMY); e: ácido fítico (PA); f: paeonol (PAE); g: propóleos (PR); h: vitamina E (VE).
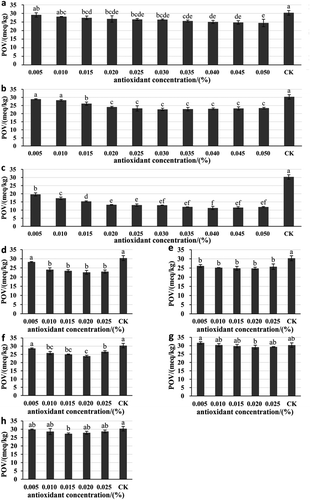
The concentration effects of natural antioxidants to inhibit lipid oxidation were reflected in . A smaller PV of TPSO sample implies a stronger antioxidant capacity of antioxidant. It could be observed that all the natural antioxidants showed antioxidant capacity compared to the control. Different concentrations showed diverse antioxidant effects. Relatively, the best antioxidant capacities were exhibited at the concentration of 0.05% (AOB), 0.03% (RE), 0.04% (TP), 0.02% (DMY), 0.02% (PA), 0.02% (PAE), 0.02% (PR), and 0.015% (VE), respectively. At optimized concentrations, all the natural antioxidants showed significant antioxidant effects in the TPSO system. Below these concentrations, the inhibitory effects of antioxidants increased as the concentration increases. Although the effectiveness of antioxidant depends on its concentration, high concentration of antioxidants may render a pro-oxidant effect (Sekhon-Loodu et al., Citation2013). The antioxidant efficiency of VE and PAE decreased, which may be caused by the imbalance of pro-oxidant and antioxidant. While, the most probable cause that led to the similar trend was the acidity of PA that may promote lipid oxidation.
3.2.2. Comparison of different antioxidants on oxidation stability of TPSO
To figure out the antioxidant influences among natural antioxidants, the comparison was made at the concentration of 0.02%. From , it could be observed that the PVs of all the treatments added antioxidants were lower than TPSO without additives. Due to the different chemical structures of antioxidants, their antioxidant mechanisms and effects were different (Taghvaei & Jafari, Citation2015). The antioxidant efficiency decreased in the following order: TBHQ > TP > DMY > PAE > RE > PA > AOB > VE > PR > BHA > BHT > blank control. Among most of the antioxidants above, their dominant bioactive ingredients were phenolic compounds and showed good antioxidants effects, which was in accordance with previous studies (Sekhon-Loodu et al., Citation2013). Apart from PR, a remarkable antioxidants effects were observed from TPSO mixed with natural antioxidants. (p < 0.05). Thereinto, TPSO samples with RE, DMY, PAE, TP, and PA showed significant higher antioxidant abilities than positive control (BHA and BHT) (p < 0.05).
Figure 3. Effects of different natural antioxidants on PV of TPSO. Column and error bar represent mean value and standard deviation, respectively. a-f Different superscript letters denote significant differences (p < 0.05). a: Antioxidants at the concentration of 0.02%; b: Antioxidants at the optimized concentration.
Figura 3. Efectos de diferentes antioxidantes naturales en el PV del TPSO. Las barras de columna y de error representan valores medios y la desviación estándar, respectivamente. a-f Las distintas letras en superíndice indican diferencias significativas (p < 0.05). a: Antioxidantes a una concentración de 0.02%; b: Antioxidantes a la concentración optimizada.
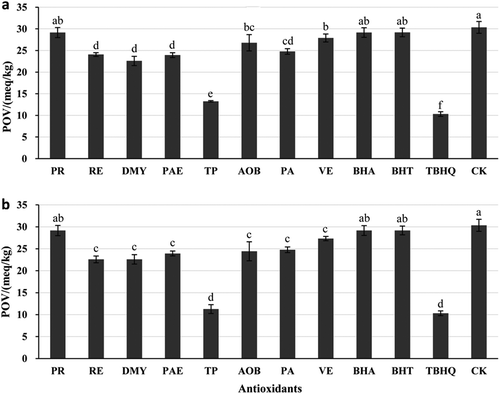
As well, the antioxidant capacity of different antioxidants at their optimized concentrations was compared and the result was showed in . We obtained a similar order of antioxidant efficiency as shown in . More interesting, the antioxidant effect of TP was almost the same as TBHQ, which is the most desirable individual natural antioxidant to substitute for synthetic antioxidants in the oil industry.
3.3. Synergistic effect of composite natural antioxidants and synergists
To explore the synergistic effect, five more effective natural antioxidants (based on our previous screening work above) and two synergists were evaluated based on PV, DPPH radical scavenging assay, and ABTS radical scavenging assay. Experimental treatments are shown in .
Table 1. Antioxidant capacity and peroxide value of TPSO mixed with composite natural antioxidants and synergists.
Tabla 1. Capacidad antioxidante e índice de peróxido del TPSO mezclado con antioxidantes naturales compuestos y sinergistas.
3.3.1. Antioxidant capacity of composite natural antioxidants on TPSO
RE, DMY, PAE, and PA were blended with the most effective natural antioxidants TP, respectively. The influence results of antioxidants during accelerated storage on PV were shown in . We observed that the PVs of blended natural antioxidants were lower compared with single antioxidant at optimized concentrations, which indicated that combined antioxidants showed a strong synergistic effect. Compared to blank control, BHA and BHT, the efficacy of composite antioxidants showed significant positive effect to inhibit lipid oxidation.
The generation of free radical can result in cell damage and free radical-mediated diseases. DPPH and ABTS radical scavenging assay were widely used to test the ability of compounds to act as free radical scavengers or hydrogen donors, and even their antioxidant activities. The antioxidant activities of natural antioxidants were evaluated by the above-mentioned method. The inhibition ability of DPPH and ABTS radicals by Trolox were given by the equation y = −0.0006x + 1.0893 (R2 = 0.9967) and y = −0.0025x + 0.4284 (R2 = 0.9986). As seen in , their antioxidant capacities were various due to different treatments. The DPPH radical scavenging activities of TP + DMY, TP + RE, TP + PAE, and TP + AOB were 99.01 mg TE/100 g, 103.01 mg TE/100 g, 79.37 mg TE/100 g, and 84.47 mg TE/100 g, respectively. It demonstrated that composite antioxidants have stronger antioxidant abilities compared with individual natural antioxidants, synthetic antioxidant, and blank control. As well, we obtained almost the similar ABTS radical scavenging effects as DPPH radical scavenging assay. Numerous studies had demonstrated that combined antioxidants can form a new redox system and regenerate each other or generate new bioactive substances, which can enhance their antioxidant capacity and exhibit remarkable synergistic effects (Hraš, Hadolin, Knez, & Bauman, Citation2000; Lasekan, Abu, & Hashim, Citation2013; Wei, Zhou, Cai, Yang, & Liu, Citation2006). As healthy promoting agents, natural combined antioxidants can be used to prevent or treat radical-related chronic diseases.
3.3.2. Antioxidant capacity of natural composite antioxidants blended with synergists on TPSO
Synergist itself has little or no significant antioxidant capacity, but it can maintain the activities of other antioxidants by reducing the primary antioxidant free radicals to achieve enhanced synergistic effect (Upadhyay & Mishra, Citation2015). Ascorbyl palmitate (AP) and citric acid (CA) are additives and conventionally used as synergists (Upadhyay & Mishra, Citation2015). Thereinto, AP is the only antioxidant that can be used in infant foods in China.. In purpose of further improving the effect of composite antioxidants, AP and CA were added and compared in the TPSO system.
The results showed that the antioxidant effects to inhibit TPSO oxidation and scavenge free radicals were enhanced to a certain extent in the present of AP and CA. The effect of AP, by contrast, was better than that of CA. That may be due to that AP is more lipid-soluble to play its role in antioxidant (Hamilton, Kalu, McNeill, Padley, & Pierce, Citation1998). Furthermore, CA acted as an organic acid and may also contribute to lipid oxidative rancidity. Among the combined natural antioxidants blended with synergists, the lipid antioxidant effects of T13, T14, T15, T16, and T20 were significantly better than synthetic antioxidant, particularly TBHQ. Thereinto, T13, namely TP + DMY + AP, showed the PV of 9.73 meq/kg and most effectively inhibited TPSO oxidation after a week in accelerated storage test.
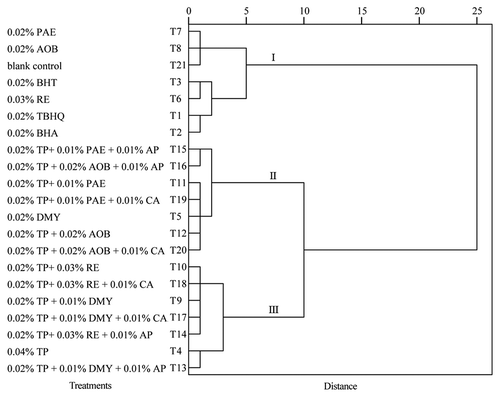
3.3.3. Hierarchical cluster analysis
Although the antioxidants of oil sample were measured based on PV, DPPH radical scavenging assay, and ABTS radical scavenging assay, their possible similarities and groupings were not impressively illustrated. Hierarchical cluster analysis (HCA) is a powerful tool to explore grouping and identifying data distributions. Three different clusters were obtained based on euclidian distance and ward’s method (). Each cluster consisted of seven treatments. Three individual natural antioxidants, all synthetic antioxidants, and blank control were clustered in cluster I. Compared with cluster II and III, most of oil samples in cluster I showed significantly higher values of PV, while significantly lower capacities in scavenging DPPH and ABTS. Obviously, composite natural antioxidants had significant synergistic effects on stabilization of edible oil, but also possessed benefit for human health.
4. Conclusion
From the present study, it is concluded that TPSO was vulnerable to oxidation affected by light, air, and temperature, which indicated TPSO is a good lipid model to study natural antioxidants. All the natural antioxidants showed certain antioxidant effects compared to TPSO without antioxidant and concentration effect. The optimum concentrations of AOB, RE, TP, DMY, PA, PAE, PR and VE were 0.05%, 0.03%, 0.04%, 0.02%, 0.02%, 0.02%, 0.02%, and 0.015%, respectively. The individual antioxidant TP exhibited most effectively on lipid preservation and free radical scavenging capacity compared to other natural antioxidants. The composite natural antioxidants of DMY, RE, PAE, and AOB blended with TP showed significant synergistic effects. The efficiency of 0.02% TP + 0.01% DMY and 0.02% TP + 0.02% AOB were comparable to TBHQ in preventing oil from oxidation. Compared with CA, AP significantly increased antioxidant efficiency and capacity of composite antioxidants. The effects of all the composite antioxidants mixed with AP were better than synthetic antioxidants. Thereinto, 0.02% TP + 0.01% DMY + 0.01% AP exhibited most outstanding. In summary, natural antioxidants are beneficial for lipid stabilization by inhibiting oxidation process and contribute to health promotion rendered by dietary nutritional supplements and functional food ingredients, which are desirable substitutes for synthetic antioxidants and have potential application prospects in food industry.
Acknowledgments
Thanks to the financial support provided by the Special Public Welfare Industry Research Projects of National Forestry Bureau, China (Project No. 201404701). The authors gratefully acknowledge all the members of national engineering technology research center for oil peony.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bera, D., Lahiri, D., & Nag, A. (2006). Studies on a natural antioxidant for stabilization of edible oil and comparison with synthetic antioxidants. Journal of Food Engineering, 74, 542–545.
- Bouaziz, M., Fki, I., Jemai, H., Ayadi, M., & Sayadi, S. (2008). Effect of storage on refined and husk olive oils composition: Stabilization by addition of natural antioxidants from Chemlali olive leaves. Food Chemistry, 108, 253–262.
- Bubonja-Sonje, M., Giacometti, J., & Abram, M. (2011). Antioxidant and antilisterial activity of olive oil, cocoa and rosemary extract polyphenols. Food Chemistry, 127, 1821–1827.
- Chang, S. S., Ostric-Matijasevic, B., Hsieh, O. A. L., & Huang, C. L. (2010). Natural antioxidants from rosemary and sage. Journal of Food Science, 42, 1102–1106.
- Chen, X., Zhang, Y., Zu, Y., Yang, L., Lu, Q., & Wang, W. (2014). Antioxidant effects of rosemary extracts on sunflower oil compared with synthetic antioxidants. International Journal of Food Science and Technology, 49, 385–391.
- Cheng, F., Li, J., & Yu, L. (1998). Exportation of Chinese tree peonies (Mudan) and their developments in other countries. II. Wild species. Journal of Northwest Normal University (Natural Science), 34, 103–107.
- Chiari, B. G., Trovatti, E., Pecoraro, É., Corrêa, M. A., Cicarelli, R. M. B., Ribeiro, S. J. L., & Isaac, V. L. B. (2014). Synergistic effect of green coffee oil and synthetic sunscreen for health care application. Industrial Crops and Products, 52, 389–393.
- Choe, E., & Min, D. B. (2006). Mechanisms and factors for edible oil oxidation. Comprehensive Reviews in Food Science and Food Safety, 5, 169–186.
- Dolatabadi, J. E. N., & Kashanian, S. (2010). A review on DNA interaction with synthetic phenolic food additives. Food Research International, 43, 1223–1230.
- Farag, R., Badei, A., & El Baroty, G. (1989). Influence of thyme and clove essential oils on cottonseed oil oxidation. Journal of the American Oil Chemists’ Society, 66, 800–804.
- Frankel, E. N. (2005). Lipid Oxidation (2nd ed.). Bridgwater, England: The Oily Press.
- Gharby, S., Harhar, H., Guillaume, D., Haddad, A., Matthäus, B., & Charrouf, Z. (2011). Oxidative stability of edible argan oil: A two-year study. LWT-Food Science and Technology, 44, 1–8.
- Guo, Q., Gao, S., Sun, Y., Gao, Y., Wang, X., & Zhang, Z. (2016). Antioxidant efficacy of rosemary ethanol extract in palm oil during frying and accelerated storage. Industrial Crops and Products, 94, 82–88.
- Hamilton, R., Kalu, C., McNeill, G., Padley, F., & Pierce, J. (1998). Effects of tocopherols, ascorbyl palmitate, and lecithin on autoxidation of fish oil. Journal of the American Oil Chemists’ Society, 75, 813–822.
- Hou, D.-X. (2003). Potential mechanisms of cancer chemoprevention by anthocyanins. Current Molecular Medicine, 3, 149–159.
- Hraš, A. R., Hadolin, M., Knez, Ž., & Bauman, D. (2000). Comparison of antioxidative and synergistic effects of rosemary extract with α-tocopherol, ascorbyl palmitate and citric acid in sunflower oil. Food Chemistry, 71, 229–233.
- Kathirvel, P., & Rupasinghe, H. V. (2011). Plant-derived antioxidants as potential omega-3 PUFA stabilizers (pp. 143–154). Hauppauge, NY, USA: Nova Science Publisher USA.
- Kim, K.-B., Nam, Y. A., Kim, H. S., Hayes, A. W., & Lee, B.-M. (2014). α-Linolenic acid: Nutraceutical, pharmacological and toxicological evaluation. Food and Chemical Toxicology, 70, 163–178.
- Knekt, P., Kumpulainen, J., Järvinen, R., Rissanen, H., Heliövaara, M., Reunanen, A., … Aromaa, A. (2002). Flavonoid intake and risk of chronic diseases. American Journal of Clinical Nutrition, 76, 560–568.
- Kolanowski, W. (2008). Seasonal variability in intake of fish oil dietary supplements among inhabitants of Warsaw. Nutrition Research, 28, 245–250.
- Lasekan, A., Abu, B. F., & Hashim, D. (2013). Potential of chicken by-products as sources of useful biological resources. Waste Management, 33, 552–565.
- Lee, S. Y., Jung, M. Y., & Yoon, S. H. (2014). Optimization of the refining process of camellia seed oil for edible purposes. Food Science & Biotechnology, 23(1), 65–73.
- Li, C., Du, H., Wang, L., Shu, Q., Zheng, Y., Xu, Y., … Ge, Y. (2009). Flavonoid composition and antioxidant activity of tree peony (Paeonia section Moutan) yellow flowers. Journal of Agricultural and Food Chemistry, 57, 8496–8503.
- Li, S. S., Wang, L. S., Shu, Q. Y., Wu, J., Chen, L. G., Shao, S., & Yin, D. D. (2015a). Fatty acid composition of developing tree peony (Paeonia section Moutan DC.) seeds and transcriptome analysis during seed development. BMC Genomics, 16, 208.
- Li, S. S., Yuan, R. Y., Chen, L. G., Wang, L. S., Hao, X. H., Wang, L. J., … Du, H. (2015b). Systematic qualitative and quantitative assessment of fatty acids in the seeds of 60 tree peony (Paeonia section Moutan DC.) cultivars by GC-MS. Food Chemistry, 173, 133–140.
- Li, X., Lin, J., Gao, Y., Han, W., & Chen, D. (2012). Antioxidant activity and mechanism of Rhizoma Cimicifugae. Chemistry Central Journal, 6, 140.
- Martínez, M. L., Penci, M. C., Ixtaina, V.,Ribotta, P. D., & Maestri, D. (2013). Effect of natural and synthetic antioxidants on the oxidative stability of walnut oil under different storage conditions. LWT - Food Science and Technology, 51(1), 44–50.
- Picerno, P., Mencherini, T., Sansone, F., Del Gaudio, P., Granata, I., Porta, A., & Aquino, R. P. (2011). Screening of a polar extract of Paeonia rockii: Composition and antioxidant and antifungal activities. Journal of Ethnopharmacology, 138, 705–712.
- Rehman, Z.-U., & Salariya, A. (2006). Effect of synthetic antioxidants on storage stability of Khoa–A semi-solid concentrated milk product. Food Chemistry, 96, 122–125.
- Sekhon-Loodu, S., Warnakulasuriya, S. N., Rupasinghe, H. V., & Shahidi, F. (2013). Antioxidant ability of fractionated apple peel phenolics to inhibit fish oil oxidation. Food Chemistry, 140, 189–196.
- Shahidi, F. (2015). Omega-3 fatty acids and marine oils in cardiovascular and general health: A critical overview of controversies and realities. Journal of Functional Foods, 797–800. doi:10.1016/j.jff.2015.09.038
- Shahidi, F., & Zhong, Y. (2010). Novel antioxidants in food quality preservation and health promotion. European Journal of Lipid Science and Technology, 112, 930–940.
- Simopoulos, A. (2006). Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed Pharmacother, 60, 502–507.
- Taghvaei, M., & Jafari, S. M. (2015). Application and stability of natural antioxidants in edible oils in order to substitute synthetic additives. Journal of Food Science and Technology, 52, 1272–1282.
- Upadhyay, R., & Mishra, H. N. (2015). A multivariate approach to optimise the synergistic blend of oleoresin rosemary (Rosmarinus officinalis L.) and ascorbyl palmitate added into sunflower oil. International Journal of Food Science & Technology, 50, 974–981.
- Upadhyay, R., & Mishra, H. N. (2016). Effect of relative humidity and light conditions on the oxidative stability of sunflower oil blends stabilised with synthetic and natural antioxidants. International Journal of Food Science & Technology, 51, 293–299.
- Wei, Q.-Y., Zhou, B., Cai, Y.-J., Yang, L., & Liu, Z.-L. (2006). Synergistic effect of green tea polyphenols with trolox on free radical-induced oxidative DNA damage. Food Chemistry, 96, 90–95.
- Wojcik, M., Burzynska-Pedziwiatr, I., & Wozniak, L. (2010). A review of natural and synthetic antioxidants important for health and longevity. Current Medicinal Chemistry, 17, 3262–3288.
- Ye, L., Wang, H., Duncan, S. E., Eigel, W. N., & O’Keefe, S. F. (2015). Antioxidant activities of Vine Tea (Ampelopsis grossedentata) extract and its major component dihydromyricetin in soybean oil and cooked ground beef. Food Chemistry, 172, 416–422.
- Young, I., & Woodside, J. (2001). Antioxidants in health and disease. Journal of Clinical Pathology, 54, 176–186.
- Zhang, Y., Wu, X., Yu, Z., Luo, D., Lu, B., & Zhang, Y. (2008). Antioxidant of bamboo leaves and its uses. United States Patent Application 20080233242.
- Zuta, P., Simpson, B., Zhao, X., & Leclerc, L. (2007). The effect of α-tocopherol on the oxidation of mackerel oil. Food Chemistry, 100, 800–807.