 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Nutritional composition, content of phenolic compounds and antioxidant properties of the four Mexican varieties of Ayocote beans (Phaseolus coccineus L.) were studied. Ayocote beans were found to be a promising source of proteins, carbohydrates, fibre and minerals. Sucrose (55.6–62.2 g/kg) and stachyose (24–24.4 g/kg) were considered as the major sugar and oligosaccharide, respectively. Glutamic acid was the most abundant amino acid (32.2 to 35.8 g/kg), while cysteine was present at the lowest concentration (2.3–2.4 g/100 kg). Purple variety contains the highest amount of total phenolic compounds (2075.9 mg GAE/kg), total flavonoids (1612.9 mg QE/kg) and total anthocyanins (1193.2 mg CGE/kg). This variety also exhibited the most effective antioxidant activities, which were evaluated by DPPH (17,040 µmol TE/kg) and ORAC (51,620 µmol TE/kg). The results obtained reveal a high potential for wider use of Ayocote bean as a remarkable source of bioactive compounds and health-promoting food.
RESUMEN
Se estudiaron la composición nutricional, contenido de compuestos fenólicos y propiedades antioxidantes de cuatro variedades de Ayocote (Phaseolus coccineus L.) mexicano. Dicho cultivo mostró ser una buena fuente de proteínas, carbohidratos, fibra y minerales. La sacarosa (55.6–62.2 g/kg) y estaquiosa (24-24.4 g/kg) se identificaron como los principales azúcar y oligosacárido, respectivamente. En cuanto a aminoácidos, el ácido glutámico (32.2-35.8 g/kg) y la cisteína (2075.9 mg GAE/kg), son el de mayor y menor abundancia, respectivamente. En la variedad morada se cuantificaron las mayores concentraciones de compuestos fenólicos totales (2075.9 mg GAE/kg), flavonoides totales (1612.9 mg QE/kg) y antocianinas totales (1193.2 mg CGE/kg), así como presentar la mayor actividad antioxidante evaluándose por DPPH (17,040 µmol TE/kg) y ORAC (5162 µmol TE/kg). Los resultados obtenidos revelan un alto potencial para un uso más amplio del frijol Ayocote como una fuente prometedora de compuestos bioactivos y alimentos que promueven la salud.
Attention towards underutilized legumes has increased remarkably as they are considered to be an alternative source of proteins with values ranging from 160 to 250 g/kg (Sánchez-Chino, Jiménez-Martínez, Dávila-Ortiz, Álvarez-González, & Madrigal-Bujaidar, Citation2015; Suárez-Martínez et al., Citation2016). In addition, legumes are beneficial for human health due to their bioactive compounds which includes phenolic acids, flavonoids and anthocyanins; these compounds are responsible for different antioxidant and antimutagenic (Cardador-Martinez, Castano-Tostado, & Loarca-Pina, Citation2002), anticarcinogenic (Díaz-Batalla, Widholm, Fahey, Castaño-Tostado, & Paredes-López, Citation2006) and anti-inflammatory activities (Oomah, Corbé, & Balasubramanian, Citation2010).
The genus Phaseolus comprises several wild and cultivated species including P. vulgaris (common bean),P. lunatus (lima bean),P. acutifolius (tepary bean), P. polyanthus (year bean) and P. coccineus (Ayocote bean) (Sousa-Sánchez & Delgado-Salinas, Citation1993). Despite the dry seeds of Ayocote bean have a nutritional content similar to related species of Phaseolus, they are only important in local diet, and consequently consumption is low and limited to the region where they are cultivated.
To exploit the health-promoting functionalities of minimally consumed legumes it is important to focus on their bioactive compounds. Although the chemical composition of P. coccineus has been reported (Mosisa, Citation2017), Ayocote bean is a crop that to our knowledge has not been fully characterized in terms of nutritional composition, content of phenolic compounds and antioxidant properties.
Ayocote beans are a natural source of nutrients even though they are considered to be an underutilized legume. Therefore, the objective of this study was to determine the nutritional (proximal analysis) and bioactive (total phenolic, flavonoid and anthocyanin contents, and the corresponding antioxidant activity) characteristics of Ayocote beans in order to highlight its health benefits. This study is also expected to contribute to promotion of its consumption as a functional food crop and as a new ingredient in the diet.
1. Materials and methods
1.1. Plant material
Four varieties of Ayocote beans were assayed in the present study. The varieties were collected from different regions of México in 2016, under local growing methods: A purple variety was collected from Yecapixtla, Morelos (18°52’41.82”N and 98°51’39.92”W), at an altitude of 1300–2200 m, an average annual temperature of 21°C and the annual rainfall is 1000 mm, on clay soil. A black variety was obtained from Ixtlahuaca de los Reyes, Edo de Mexico (19°34’51” N and 99°46’81” W), at an altitude of 2300–4000 m, an average annual temperature of 14°C and the annual rainfall is 950 mm, on dark soil rich in organic matter. A brown variety cultivated in Chiautla de Tapia, Puebla (18°17’58.91’’ N and 98° 36’7.70’’W), at an altitude of 1000–1600 m, an average annual temperature of 2°C and an annual rainfall of 800 mm, on stony soils rich in organic matter. A white variety harvested in Sombrerete, Zacatecas, (23°38’3.28”N and 103°38’21.00” W), at an altitude of 2000–3100 m, an average annual temperature of 21°C and an annual rainfall of 1000 mm, on stony soils rich in organic matter.
Seeds from the four Ayocote beans were dried in the sun to a water content of ca. 10%. Dried seeds of each variety were ground to flour with an IKA all basic mill (IKAWorks, Inc., Wilmington, NC) to pass through a 40-mesh sieve. The Ayocote bean powders were stored at −20°C until further analysis.
2. Sample preparation
2.1. Crude extract
Five grams of each dried seed powder were incubated in a conical centrifuge tube (50 mL) with 20 mL of distilled water in an orbital shaker (Lab-Line Orbit Environ, Model 3527, Melrose Plaza, IL) at 200 rpm, room temperature, for 6 h in the absence of light. After incubation, the samples were centrifuged at 14 308 × g for 15 min; the supernatant was collected and stored at −20°C and used in further experiments. Extracts were prepared in triplicate (n = 3) for each Ayocote variety.
2.2. Chemical composition
Total protein content was determined by micro-Kjeldahl (AOAC 960.52). Moisture, ash, lipids and fibre were analysed according to AOAC methods 945.38, 930.03, 920.30 and 985.28 (AOAC, Citation1995), respectively, Total carbohydrates were calculated by difference: Total carbohydrates = 100 – (g moisture + g protein + g lipids + g ash).
2.3. Mineral composition
The specific minerals present in the Ayocote beans were analysed by inductively coupled plasma atomic absorption emission Spectrophotometer (ICP-AES), following the AOAC method 948.27 (AOAC, Citation1995), from the ash residue dissolved in acid using an Ai Spectroflaeme D (ArICP, CT, USA). Potassium and sodium were detected by emission at wavelengths of 589.6 and 769.9 nm, respectively, while calcium, magnesium, iron, zinc, copper and manganese were determined by absorption at wavelengths of 422.7, 285.2, 248.3, 213.9, 324.7 and 279.5 nm, respectively. Results were expressed as milligrams per kilogram of sample (mg/kg) based on the calibration curves prepared with standards of each mineral.
2.4. Sugar analyses
Free sugars were determined according to Barros, Cruz, Baptista, Estevinho, and Ferreira (Citation2008). The crude extract was analysed using an HPLC system (Agilent 1100), equipped with an RI detector and with a Supelco C610H column (30 cm × 7.8 mm). The mobile phase was phosphoric acid (0.1%) at a flow rate of 0.5 mL/min and the temperature was set at 40°C. The results are expressed in mg/kg of sample, calculated by internal normalization of the chromatographic peak area. Sugars were identified by comparing their retention times with those of standards.
Oligosaccharides were determined following the method reported by Smiricky et al. (Citation2002) using a Dionex DX-300 HPLC (Dionex Corp., Sunnyvale, CA) fitted with a CarboPac PA-1 (4 × 250 mm) analytical column. Galactooligosaccharides were detected using a Dionex pulsed electrochemical detector equipped with a gold working electrode. The standard curves were determined with the use of raffinose, stachyose and verbascose (Sigma-Aldrich, St. Louis, MO, USA).
2.5. Amino acid analyses
Amino acid profile of samples was quantified using a Waters ion-exchange chromatograph (Waters Corporation, Milford, MA.). The crude extract was hydrolysed in 6 M HCl for 24 h at 110°C. For measuring sulphur-containing amino acids (cysteine and methionine), the crude extract was first oxidized with performic acid prior to hydrolysis with 6 M HCl (Xu & Hanna, Citation2011). The amino acid analysis was limited to the 12 amino acids for which we had standards.
2.6. Determination of total phenolic content (TPC)
TPC was determined according to the protocol reported by Singleton, Orthofer, and Lamuela-Raventos (Citation1999), with slight modifications. Briefly, the procedure consisted of mixing 15 μL of crude extracts, 240 μL of distilled water, and 15 μL of Folin-Ciocalteu reagent in a 96-well Costar® flat bottom microplate. The mixture was incubated for 3 min, and then 30 μL of 4 N [2 M] Na2CO3 was added and incubated at room temperature for 2 h in the condition. After incubation, the absorbance was measured at 725 nm using a 96-well using a Synergy HT spectrophotometer (Synergy HT, Bio-Tek Instruments, Inc., Winooski, Vt., USA). The calculations were performed using a Gallic acid standard curve (from 0 to 0.4 mg/mL) and the content of total phenolic compounds in each extract was expressed as milligram of gallic acid equivalent/kg (mg GAE/kg).
2.7. Determination of total flavonoid content (TFC)
TFC was determined according to a colorimetric method described by Chang, Yang, Wen, and Chern (Citation2002), with slight modifications. 20 µL of the crude extract were mixed with 112 µL of water and 60 µL of methanol (80% in water) into a 96-well microplate, then 4 µL of 10% AlCl3 and 4 µL of 1 M C2H3KO2 were added. The mixture was incubated for 30 min, and the absorbance was measured at 415 nm using a Microplate Reader. A quercetin curve (from 0 to 0.4 mg/mL) was used to calculate concentration and TFC for each extract was calculated and expressed as milligram of quercetin equivalent/kg (mg QE/kg).
2.8. Determination of total anthocyanins (TA)
Analysis of TA was carried out according to Abdel-Aal and Hucl (Citation1999) with some modifications, by measuring the absorbance of ethanolic extracts at pH 1.0. One gram of dried seeds powder was homogenized in a 50 mL centrifuge tube with 25 mL of an acid–ethanol solution (0.225 mol equivalent/L HCl in ethanol:water (95:5, v/v)). The tube was flushed with nitrogen gas, agitated for 30 min and then centrifuged at 3000 × g (Sorvall RC5C, Sorvall Instruments, Dupont, Wilmington, DE) for 15 min and the supernatants collected. Absorbance readings at 535 nm were monitored and corrected for background absorbance at 700 nm (due to turbidity) in a photodiode array spectrophotometer.
Anthocyanins content was calculated as follows:
Where: C is the concentration of total anthocyanins, ε is the molar extinction coefficient of 25,965 M−1 cm−1 and MV is the molecular weight of 449.2 g/mol.
Anthocyanins were expressed as mg of cyanidin-3 glucoside equivalents/kg (mg CGE/kg).
3. Antioxidant capacity assays
3.1. Determination of DPPH radical scavenging
DPPH radical scavenging assay was carried out according to Huang, Ou, and Prior (Citation2005), with slight modifications. Briefly, a 20 µL of crude extract was placed into a 96-well microplate, then 280 µL of DPPH was added and allowed to incubate in a darkroom for 30 min at a room temperature. After that, the decrease in absorbance at 540 nm was determined in a microplate reader. The Trolox curve from 0.05 to 1 micromole TE/g was used to calculate the results, which are expressed as micromole of Trolox equivalent/kg (µmol TE/kg).
3.2. Determination of oxygen radical absorbance capacity (ORAC) assay
The assay was carried out using AAPH (2,2-azobis (2-amidino-propane) dihydrochloride) as a peroxyl radical generator, fluorescein as the fluorescent probe and Trolox as a standard according to the method of Huang, Ou, Hampsch-Woodill, Flanagan, and Prior (Citation2002). The reaction mixture contained 25 μL of crude extract, 25 μL of 75 mM phosphate buffer (pH 7.4), 75 μL of 0.8 M AAPH, and 200 μL of 0.106 μM fluorescein. The 75 mM phosphate buffer was used as a blank. The extracts, phosphate buffer and fluorescein were pre-incubated at 37°C for 15 min, after that time AAPH was added to start the reaction, and the fluorescence was measured every 70 s during 70 min with a 485 nm excitation filter and a 580 nm emission filter using a Synergy HT spectrophotometer. The values were calculated using a regression equation describing the relationship between the Trolox concentration and the net area under the fluorescein decay curve. The Trolox curve from 6.25 to 125 (μmol TE/g) was used to calculate the results, which are expressed as micromole of Trolox equivalent/kg (µmol TE/kg).
3.3. Statistical analysis
All experiments were performed in triplicate. The results were expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) followed by the Duncan’s multiple range test was used to compare the means among different groups using PASW Statistics 18.0 software (IBM, Armonk, NY, USA). Differences were considered significant at p < 0.05. The correlation analysis among phenolics, flavonoids, anthocyanins, DPPH, ORAC and minerals was performed with Pearson’s correlation test.
4. Results and discussion
4.1. Proximate composition
shows the results of proximate composition of the four Ayocote beans varieties. Moisture content ranging from 79.8 to 87.6 g/kg, is higher than the value of 71.4 g/kg previously reported by Mosisa (Citation2017) but lower than the value reported by Bernardino-Nicanor et al. (Citation2017) (103 g/kg) in different varieties of P. coccineus. From the results in this study, the brown variety showed the highest moisture value, followed by black, white and purple varieties. In relation to ash content, the average value was 4.11 g/kg, higher than that reported for P. coccineus from Colombia by Álvarez Salas and Turbay Ceballos (Citation2009) (24.1 g/kg), and lower than the data showed by Strauta, Muizniece-Brasava, and Alsina (Citation2013), who reported values among 45 to 48 g/kg in coloured P. coccineus from Latvia. The differences in ash content might be attributed to characteristics of the soil where the species were cultivated and also to the agronomic variety.
Table 1. Proximate composition of the four varieties of Mexican Ayocote (Phaseolus coccineus L.) (mg/kg). All values are mean ± standard deviation of three replicates. Means in the same row with different superscripts differ significantly (p < 0.05).
. Composición proximal delas cuatro variedades de Ayocote Mexicano (Phaseolus coccineus L.) (mg/kg). Todos los valores son media ± desviación estándar de tres repeticiones. Las medias en la misma fila con diferentes superíndices difieren significativamente (P < 0.05).
Protein content in the species assayed ranged from 180.7 to 189.3 g/kg. The protein content in this study is comparable to the values of 167 to 272 g/kg, reported by Baptista et al. (Citation2017) in dry common bean varieties from Mozambique, but lower than the values of 217–238 g/kg for different Ayocote bean varieties from México (Bernardino-Nicanor et al., Citation2017). However, it should be noted that the protein content in edible legumes could vary significantly among cultivars of a single species (Bourges, Citation1987). The difference in protein content is associated with the fertilizer application and the growing locations, which influences the yield and protein concentration of some seeds (Malik, Holm, & Johansson, Citation2012).
In accordance with the general low lipid content of legumes, values determined in the four Ayocote beans varieties were low. The brown, purple, black and white varieties did not show any significant differences (P > 0.005). The lipid content was higher compared to that observed by Mosisa (Citation2017) (9.0 g/kg) but lower than data recorded previously by Bernardino-Nicanor et al. (Citation2017) which ranged from 32.5 to 37.8 g/kg. In Mayocoba bean (P. vulgaris) lipid values of 19.8 g/kg have been reported (Osorio-Díaz, Tovar, Paredes‐López, Acosta‐Gallegos, & Bello‐Pérez, Citation2005). Other authors have reported lipid contents of 12 g/kg in different Mexican black bean varieties (Vargas-Torres et al., Citation2004); this level is close to the values obtained in the current research.
The concentration of fibre was in the ranges of 65.3 to 67.2 g/kg and significant differences (p < 0.05) among varieties were observed. The total fibre level was higher compared to other reports for Phaseolus species. Adeparusi (Citation2001) reported values of 46 g/kg in P. lunatus, whereas values from 40 to 44 g/kg and 39.2 to 44 g/kg in different varieties of P. coccineus (Mosisa, Citation2017) and P. acutifolius (Porch et al., Citation2017), respectively, were determined. The carbohydrate content ranged from 670.9 to 677.6 g/kg. These values are higher than those found by Mosisa (Citation2017) in a study on black climbing beans (P. coccineus) (658.5 g/kg), and by Aremu, Olaofe, Basu, Abdulazeez, and Acharya (Citation2010) who reported values of 531 g/kg in brown Ayocote beans from Nigeria. The carbohydrate content in the Ayocote seeds is close to the range of carbohydrates content in common beans (642.8 and 671.4 g/kg) (Fan & Beta, Citation2016) and could be a good source of energy. Some significant differences in composition were found which could be due to different cultivars, environmental conditions, and soil characteristics.
4.2. Mineral analysis
Minerals are necessary for human nutrition and are essential for a balanced diet that allows for the development of normal metabolic functions of the human body. The ratio of Na and K in the human body is important for the prevention of high blood pressure. A Na:K ratio less than 1 is recommended. Na and K are required to maintain osmotic balance of the body fluids, the pH of the body, to regulate muscle and nerve irritability, control glucose absorption and enhance normal retention of protein during growth (National Research Council, Citation1989).
shows the content of macrominerals including potassium (K), calcium (Ca), magnesium (Mg) and sodium (Na), as well as of microminerals, such as iron (Fe), zinc (Zn), manganese (Mn) and copper (Cu) in the four varieties of Ayocote bean. Mineral concentrations of the assayed beans in this study were lower than those reported previously by Aremu et al. (Citation2010) and Hels et al. (Citation2004) in P. coccineus from Nigeria and Bangladesh, respectively. They are also completely different from those reported for other Phaseolus species such as P. vulgaris, P. angulatus and P. lunatus (Suárez-Martínez et al., Citation2016). The results of the present analysis demonstrated that except for Cu in microelements and Ca in macroelements, there was a significant variation (p <0.05) in all of the other minerals found in the Ayocote beans, which might be attributed to the genetic composition of each variety and the variable uptake of minerals, as a result of different soil conditions at different habitats.
Table 2. Mineral composition of Mexican Ayocote beans (Phaseolus coccineus L.) (mg/kg). All values are mean ± standard deviation of three replicates. Means in the same row with different superscripts differ significantly (P < 0.05).
. Composición de minerales de Ayocote Mexicano (Phaseolus coccineus L.) (mg/kg). Todos los valores son media ± desviación estándar de tres repeticiones. Las medias en la misma fila con diferentes superíndices difieren significativamente (P < 0.05).
The relationships among different minerals could help to understand the absorption transport, and function in the plant. Mg was positively associated with K (Supplementary Table 1). These results could probably reflect the fact that Mg regulates the uptake of the K (Tucker, Citation1999). These results were comparable to previous studies showing a positive relationship between Mg and K (Jiang, Wu, Feng, Yang, & Shi, Citation2007; Shao et al., Citation2018).
4.3. Sugar analysis
The concentration of sugars is depicted in . Stachyose and raffinose were similar in the four varieties assayed. The results showed that the predominant sugar was sucrose.
Table 3. Sugar analyses (g/kg) in the seeds of Mexican Ayocote beans (Phaseolus coccineus L.). All values are mean ± standard deviation of three replicates. Means in the same row with different superscripts differ significantly (P < 0.05).
. Analisis de azucares (g/kg) en semillas de Ayocote Mexicano (Phaseolus coccineus L.). Todos los valores son media ± desviación estándar de tres repeticiones. Las medias en la misma fila con diferentes superíndices difieren significativamente (P < 0.05).
Stachyose was the main α-galactoside followed by raffinose and verbascose in all the cultivars assayed and their levels are within the range reported in different common bean cultivars (Porch et al., Citation2017), and higher compared to other reports (Campos-Vega et al., Citation2009). In this study, the relative content of stachyose was 24.3 g/kg, followed by raffinose (3.37 g/kg) and verbascose (0.43 g/kg). For most food legume species, the predominant oligosaccharide is stachyose (∼80%), followed by raffinose (<20%) and verbascose is very low (<10%) (Fan, Zang, & Xing, Citation2015). All Ayocote beans varieties have more relative content of sucrose (>55%) when comparing oligosaccharides stachyose (>30%), only 7–8% raffinose and a trace of verbascose (1–2%). The average of total α-galactosides in the samples of Ayocote beans assayed is 32.1 g/kg, which is within the range reported by P. vulgaris (26 and 66 g/kg of sample) (Guillon & Champ, Citation2002).
4.4. Aminoacid analysis
The amino acid contents of the four Ayocote beans seeds are summarized in . Overall, 12 amino acids were determined in each sample, six essential and six non-essential. The high contents of glutamic and aspartic acids are related to their importance as starting compounds from which the backbones of the other amino acids are formed (Viola & Anekwe, Citation2001). Leucine was the most concentrated (17.4 to 18.4 g/kg) essential amino acid in all the samples, while glutamic acid was the most concentrated nonessential amino acid (32.2 to 35.8 g/kg) in the raw sample, the limiting amino acids in seeds of the four varieties were the sulphur-containing amino acids (cysteine and methionine, 2.3 and 2.8 g/kg, respectively) as it is expected in legumes (Olaofe, Umar, & Adediran, Citation1993).
Table 4. Amino acid profile (g/kg) for the four varieties of Mexican Ayocote (Phaseolus coccineus L.). All values are mean ± standard deviation of three replicates. Means in the same row with different superscripts differ significantly (P < 0.05).
. Perfil de aminoacidos (g/kg) de las cuatro variedades de Ayocote Mexicano (Phaseolus coccineus L.). Todos los valores son media ± desviación estándar de tres repeticiones. Los medios en la misma fila con diferentes superíndices difieren significativamente (P < 0.05).
Different values of amino acids in cranberry bean (Phaseolus coccineus L.) were reported by Aremu et al. (Citation2010), although with a similar tendency in the content of amino acids, this behaviour could be attributed to the agronomic differences and species studied.
The levels in sulphur-containing amino acids in Ayocote beans can be compensated by mixing them with cereals which have rich contents of methionine and cysteine besides, legumes provide the lysine which the cereals lack. Complementing the amino acid content of Ayocote with cereals, it is possible to achieve a balance of amino acids of vegetable source.
4.5. Total phenolic compounds (TPC)
Phenolic compounds contribute to the overall antioxidant activities of plant foods, removing free radicals, chelating metal catalysts, activating antioxidant enzymes, and inhibiting oxidases.
The total phenolic compounds in seeds from the different cultivars of Ayocote bean were significantly different and decreased in the following order purple>black>brown>white (). Generally, earlier comparative studies conducted on different bean varieties demonstrated that the total phenolic content in white beans was lower than in some coloured beans (Djordjevic, Śiler-Marinkovic, & Dimitrijevic-Brankovic, Citation2011; Orak, Karamać, Orak, & Amarowicz, Citation2016). TPC determined for each cultivar was found to be comparable to those reported in pigmented beans (P. vulgaris L.) from México ranging from 900 to 2110 mg of GAE/kg (Espinosa-Alonso, Lygin, Widholm, Valverde, & Paredes-Lopez, Citation2006), other studies mentioned close TPC values: 1123–1940 mg of GAE/kg in Mexican beans and the ranges reported by Rocha-Guzmán, González-Laredo, Ibarra-Pérez, Nava-Berumen, and Gallegos-Infante (Citation2007) overlapped, and were 1550 to 5460 mg of GAE/kg. Total phenolic contents in beans differ among studies and could be attributed to various factors such as genotype, agronomic practices, maturity at harvest, storage condition beside on the solvents used during extraction.
Table 5. Total phenolic content, total flavonoids and total anthocyanins for the four varieties of Mexican Ayocote (Phaseolus coccineus L.). All values are mean ± standard deviation of three replicates. Means in the same column with different superscripts differ significantly (p < 0.05).
. Contenido de compuestos fenólicos, flavonoides y antocianinas totales de las cuatro variedades de Ayocote Mexicano (Phaseolus coccineus L.). Todos los valores son media ± desviación estándar de tres repeticiones. Las medias en la misma columna con diferentes superíndices difieren significativamente (P < 0.05).
4.6. Total flavonoid content (TFC)
Flavonoids are widespread plant secondary metabolites. As components of vegetables and fruits, they are regularly contained in foods for human. However, in our literature review, we did not find reports about total flavonoid content in Ayocote bean. Quiróz-Sodi, Mendoza-Díaz, Hernández-Sandoval, and Carrillo-Ángeles (Citation2018) reported the presence of the flavonoids catechin, apigein and kaemferol in three types of Ayocote beans from Mexico but not the total flavonoids. In this study significant differences (p < 0.05) in TFC were found among the cultivars () ranged from 1084.5 for white to 1612.9 mg/kg for purple variety. The results were similar to those reported by Mojica, Meyer, Berhow, and de Mejía (Citation2015) who refer a range from 1240 to 1430 mg QE/kg and higher than the findings cited by Rocha-Guzmán et al. (Citation2007) (83 to 980 mg QE/kg) in beans (P. vulgaris) of different pigmentation. Growing location, year, post-harvest store, and the species of Phaseolus evaluated might contribute to the variation content in flavonoids.
4.7. Total anthocyanins content (TAC)
Polyphenolic compounds such as anthocyanins are able to act as radical scavengers and thus possess a strong antioxidant activity (Lopez-Martinez et al., Citation2009). TAC ranged from 2.2 to 1193.2 mg CGE/kg (). The samples showed important differences. The highest and lowest values of TA were found in purple and white seed coats, respectively. Thus, for the varieties examined, those that were most abundant in total phenolic content were also most abundant in anthocyanins (which contribute to the total phenolic levels). In accordance to previous findings (Akond et al., Citation2011), where 29 genotypes of pigmented common beans were explored and coloured beans showed the highest content of anthocyanins, followed by brown and finally by cream beans. We measured a low concentration of anthocyanins in white variety as anthocyanins are a group of pigments that contribute significantly to red-blue colouration in plants material. Results obtained were somewhat similar to those reported in Mexican bean cultivars (10 to 1850 mg CGE/kg) (Espinosa-Alonso et al., Citation2006) and higher (370–710 mg CGE/kg) than the ones determined by Salinas-Moreno, Rojas-Herrera, Sosa-Montes, and Pérez-Herrera (Citation2005). In a recent study, Quiróz-Sodi et al. (Citation2018) studied the total anthocyanin content in three types of Ayocote beans from Mexico, they reported values of 440 mg CGE/kg to 1220 mg CGE/kg, which are higher than the values reported in this study; this may be due to the fact that the seeds were mainly black spotted with brown.
4.8. Antioxidant activities
It has been found that polyphenolic compounds are one of the most effective antioxidant constituents in plant foods; including legumes. Thus, it is important to quantify polyphenolic contents and to assess their contribution to antioxidant activity as we discuss next.
DPPH quenching activity of the samples of the four Ayocote bean varieties is shown in . DPPH radical scavenging varied from 12,200 to 17,040 µM TE/kg. Significant differences (P < 0.05) in DPPH existed among ayocote beans cultivars. The highest radical scavenging activity was exhibited by the purple variety followed by black, brown and white varieties. Thus, varieties exhibiting greatest DPPH quenching capacity appeared to be those with the greatest proportion of anthocyanins. These results are consistent with those of Akond et al. (Citation2011) who reported that genotypes with dark coloured testa showed relatively higher levels of antioxidant activity in highly pigmented common beans. The differences in the antioxidant capacity among coloured phenotypes are possibly related to the specific composition of anthocyanin derivatives such as simple or acylated glycosides of cyanidin, pelargonidin or peonidin (Xu, Yuan, & Chang, Citation2007; Choung, Choi, An, Chu, & Cho, Citation2003; Mojica et al., Citation2015)
Figure 1. Antioxidant activity of the four Mexican varieties of Ayocote beans (Phaseolus coccineus L.). Means in the same antioxidant activity with different superscripts differ significantly (p < 0.05).
. Actividad antioxidante de cuatro variedades de ayocote (Phaseolus coccineus L.). Medias en la misma actividad antioxidante con diferentes letras superscript difieren significantemente (p < 0.05).
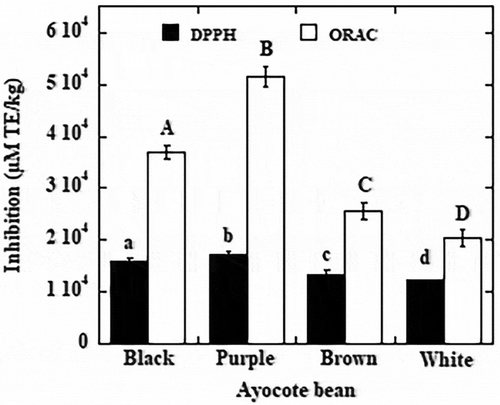
Wołosiak, Drużyńska, Piecyk, Majewska, and Worobiej (Citation2018) studied a variety of Ayocote cv. Ja´s Karłowy from Poland and reported value of antioxidant activity of 16,884 µM TE/kg which is within the reported range in this study. Compared to DPPH values in different varieties of pigmented raw beans (1230 to 5900 μM TE/g and 1490 to 1845 μM TE/kg (Xu & Chang, Citation2007; Ranilla, Genovese, & Lajolo, Citation2007), respectively, Ayocote beans showed higher antioxidant activity. However, our results were lower than those reported by Xu and Chang (Citation2007), which ranged from1480 in navy beans to 18,950 μmol of TE/g in black turtle beans.
Generally, the same variety showing greatest DPPH scavenging activity were also those with greatest ORAC activity (), the highest activity (51,620 μmol TE/kg sample) was attained by purple Ayocote bean, followed by black (36,940 µmol TE/kg), brown (25,570 μmol TE/kg) and white (20,310 μmol TE/kg) and this behaviour could be due to the profile of phenolic compounds (including anthocyanins) in each of the varieties of ayocote.
Our values were lower than those previously reported in black beans by Xu and Chang (Citation2007) (from 21,400 to 79,270 μmol TE/kg). Ayocote beans showed higher ORAC value than those reported in other studies, Oomah et al. (Citation2010) found values which ranged from 4100 to 7250 µM TE/kg in beans of different pigmentation, common beans grown in Central Malawi showed values of ORAC from 1800 to 3054 μmol TE/kg (Fan & Beta, Citation2016) and two Spanish varieties (1313 to 1604 µmol TE/kg) (Pedrosa et al., Citation2015).
The different results achieved are due to the antioxidant methods utilized, as well as the agronomic conditions and to species evaluated.
Correlations significantly positive were observed among the TPC, TF, TA, DPPH and ORAC and between DPPH and ORAC (Supplementary Table 2).
The variety most enriched in phenolic compounds (purple) yielded the most effective antioxidant activity of the samples examined () and this result confirms that the phenolic compounds may be responsible for the major portion of the antioxidant activity of the Ayocote beans.
It is worth noting that pigmented grains exhibited higher antioxidant activities. Those higher values in DPPH and ORAC are related to the presence of phenolic compounds such as anthocyanins and thus, in three of the four Ayocote beans assayed showed higher values than the white variety. However, other non-coloured antioxidant compounds such as quercitin, catechin, ferulic acid, sinapic acid and its derivatives are present in white beans (García-Lafuente et al., Citation2014).
Correlations significantly positive were observed among the TPC, TF, TA, DPPH and ORAC and between DPPH and ORAC (Supplementary Table 2). Previous studies have reported the contribution of total phenolic content to the antioxidant capacity of legume extracts (Zhang et al., Citation2015; Zhao, Du, Wang, & Cai, Citation2014). The analysis of the correlation between the antioxidant activities (DPPH and ORAC) and phenolic contents (TPC, TFC and TA) using the Pearson’s correlation coefficient showed strong correlations. TPC, TFC, TA and DPPH presented correlations (R2 = 0.95), TPC (R2 = 0.98) and TA (R2 = 0.97). The antioxidant activity as measured by the ORAC assay positively and strongly correlated, TPC, TFC and TA, with correlation coefficients at R2 = 0.98, 0.98 and 0.93 and 0.98, 0.96, 0.93, respectively.
These results suggest that phenolic compounds are major contributors to the antioxidant activity of Ayocote beans.
In general, studies regarding the content of total phenolic compounds and antioxidant activity in Ayocote beans are scarce.
4.9. Conclusion
The genus Phaseolus has received considerable attention for its content of phytochemicals and nutritional value. The present study highlighted Ayocote beans as a promising source of valuable nutritional components, rich in dietary antioxidants and other micronutrients. All varieties were characterized by low lipid and high content of carbohydrates. Significant differences were found in all minerals except Ca and Cu in each of the varieties. Sucrose and stachyose were considered as the major sugar and oligosaccharide. Leucine was the most concentrated essential amino acid, while glutamic acid was the most concentrated non essential amino acid in all the samples. Significant differences were found among the four varieties, in relation to phenolic compounds (total phenolic content, total flavonoids and total anthocyanins) and antioxidant properties. Purple variety had the highest content of phenolic compounds and antioxidant activity. Studies must be conducted to preserve the Mexican biodiversity which includes this crop, which is potentially exploitable as a foodstuff. Further work is important to determinate the effect of processing methods on chemical composition and antioxidant activity.
Supplemental Material
Download Zip (20.2 KB)Acknowledgments
Authors gratefully acknowledge to Manuel Bernal for the technical support at Centro de Investigation en Alimentation y Desarrollo, CIAD, A.C. Culiacan, Sin. Mexico.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Abdel-Aal, E. S., & Hucl, P. (1999). A rapid method for quantifying total anthocyanins in blue aleurone and purple pericarp wheats. Cereal Chemistry, 76, 350–354.
- Adeparusi, E. O. (2001). Effect of processing on the nutrients and anti‐nutrients of lima bean (Phaseolus lunatus L.) flour. Food/Nahrung, 45, 94–96.
- Akond, A. S. M. G. M., Khandaker, L., Berthold, J., Gates, L., Peters, K., Delong, H., & Hossain, K. (2011). Anthocyanin, total polyphenols and antioxidant activity of common bean. American Journal of Food Technology, 6, 385–394.
- Álvarez Salas, L. M., & Turbay Ceballos, S. (2009). El fríjol petaco (Phaseolus coccineus) y la maravilla (Phaedranassa sp.): Aspectos etnobotánicos de dos plantas alimenticias de origen americano en el Oriente antioqueño, Colombia. Agroalimentaria, 15, 101–113.
- AOAC. (1995). Official methods of analysis of AOAC international (17th ed.). Gaithersburg, MD, USA: Association of Official Analytical Chemists, Arlighton.
- Aremu, M. O., Olaofe, O., Basu, S. K., Abdulazeez, G., & Acharya, S. N. (2010). Processed cranberry bean (Phaseolus coccineus L.) seed flour for the African diet. Canadian Journal of Plant Science, 90, 719–728.
- Baptista, A., Pinho, O., Pinto, E., Casal, S., Mota, C., & Ferreira, I. M. (2017). Characterization of protein and fat composition of seeds from common beans (Phaseolus vulgaris L.), cowpea (Vigna unguiculata L. Walp) and bambara groundnuts (Vigna subterranea L. Verdc) from Mozambique. Journal of Food Measurement and Characterization, 11(2), 442–450.
- Barros, L., Cruz, T., Baptista, P., Estevinho, L. M., & Ferreira, I. C. (2008). Wild and commercial mushrooms as source of nutrients and nutraceuticals. Food and Chemical Toxicology, 46, 2742–2747.
- Bernardino-Nicanor, A., Acosta-García, G., Güemes-Vera, N., Montañez-Soto, J. L., de Los Ángeles Vivar-Vera, M., & González-Cruz, L. (2017). Fourier transform infrared and Raman spectroscopic study of the effect of the thermal treatment and extraction methods on the characteristics of Ayocote bean starches. Journal of Food Science and Technology, 54, 933–943.
- Bourges, R. H. (1987). Las leguminosas en la alimentación. II Parte. Publicación del Instituto Nacional de la Nutrición, CONASUPO y sus Empresas Industriales. Cuadernos de Nutrición, 10, 22–30.
- Campos-Vega, R., Reynoso-Camacho, R., Pedraza-Aboytes, G., Acosta-Gallegos, J. A., Guzman-Maldonado, S. H., Paredes-Lopez, O., … Loarca-Piña, G. (2009). Chemical composition and in vitro polysaccharide fermentation of different beans (Phaseolus vulgaris L.). Journal of Food Science, 74(7). doi:10.1111/j.1750-3841.2009.01292.x
- Cardador-Martinez, A., Castano-Tostado, E., & Loarca-Pina, G. (2002). Antimutagenic activity of natural phenolic compounds present in the common bean (Phaseolus vulgaris) against aflatoxin B1. Food Additives & Contaminants, 19, 62–69.
- Chang, C. C., Yang, M. H., Wen, H. M., & Chern, J. C. (2002). Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of Food and Drug Analysis, 10, 3.
- Choung, M. G., Choi, B. R., An, Y. N., Chu, Y. H., & Cho, Y. S. (2003). Anthocyanin profile of Korean cultivated kidney bean (Phaseolus vulgaris L.). Journal of Agricultural and Food Chemistry, 51, 7040–7043.
- Díaz-Batalla, L., Widholm, J. M., Fahey, G. C., Castaño-Tostado, E., & Paredes-López, O. (2006). Chemical components with health implications in wild and cultivated Mexican common bean seeds (Phaseolus vulgaris L.). Journal of Agricultural and Food Chemistry, 54, 2045–2052.
- Djordjevic, T. M., Šiler-Marinkovic, S. S., & Dimitrijevic-Brankovic, S. I. (2011). Antioxidant activity and total phenolic content in some cereals and legumes. International Journal of Food Properties, 14, 175–184.
- Espinosa-Alonso, L. G., Lygin, A., Widholm, J. M., Valverde, M. E., & Paredes-Lopez, O. (2006). Polyphenols in wild and weedy Mexican common beans (Phaseolus vulgaris L.). Journal of Agricultural and Food Chemistry, 54, 4436–4444.
- Fan, G., & Beta, T. (2016). Proximate composition, phenolic profiles and antioxidant capacity of three common bean varieties (Phaseolus Vulgaris L.). Journal of Food Chemistry and Nanotechnology, 2, 2016–2023.
- Fan, P. H., Zang, M. T., & Xing, J. (2015). Oligosaccharides composition in eight food legumes species as detected by high‐resolution mass spectrometry. Journal of the Science of Food and Agriculture, 95, 2228–2236.
- García-Lafuente, A., Moro, C., Manchón, N., Gonzalo-Ruiz, A., Villares, A., Guillamón, E., … Mateo-Vivaracho, L. (2014). In vitro anti-inflammatory activity of phenolic rich extracts from white and red common beans. Food Chemistry, 161, 216–223.
- Guillon, F., & Champ, M. J. (2002). Carbohydrate fractions of legumes: Uses in human nutrition and potential for health. British Journal of Nutrition, 88, 293–306.
- Hels, O., Larsen, T., Christensen, L. P., Kidmose, U., Hassan, N., & Thilsted, S. H. (2004). Contents of iron, calcium, zinc and β-carotene in commonly consumed vegetables in Bangladesh. Journal of Food Composition and Analysis, 17, 587–595.
- Huang, D., Ou, B., Hampsch-Woodill, M., Flanagan, J. A., & Prior, R. L. (2002). High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. Journal of Agricultural and Food Chemistry, 50, 4437–4444.
- Huang, D., Ou, B., & Prior, R. L. (2005). The chemistry behind antioxidant capacity assays. Journal of Agricultural and Food Chemistry, 53, 1841–1856.
- Jiang, S. L., Wu, J. G., Feng, Y., Yang, X. E., & Shi, C. H. (2007). Correlation analysis of mineral element contents and quality traits in milled rice (Oryza stavia L.). Journal of Agricultural and Food Chemistry, 55, 9608–9613.
- Lopez-Martinez, L. X., Oliart-Ros, R. M., Valerio-Alfaro, G., Lee, C. H., Parkin, K. L., & Garcia, H. S. (2009). Antioxidant activity, phenolic compounds and anthocyanins content of eighteen strains of Mexican maize. LWT-Food Science and Technology, 42, 1187–1192.
- Malik, A. H., Holm, L., & Johansson, E. (2012). Soil and starter fertilizer and its effect on yield and protein composition of malting barley. Journal of Soil Science and Plant Nutrition, 12, 835–849.
- Mojica, L., Meyer, A., Berhow, M. A., & de Mejía, E. G. (2015). Bean cultivars (Phaseolus vulgaris L.) have similar high antioxidant capacity, in vitro inhibition of α-amylase and α-glucosidase while diverse phenolic composition and concentration. Food Research International, 69, 38–48.
- Mosisa, M. T. (2017). Effect of processing on proximate and mineral composition of black climbing (P. coccineus L.) bean flour. African Journal of Food Science, 11, 74–81.
- National Research Council. (1989). Improving risk communication. Washington, DC: National Academies.
- Olaofe, O., Umar, Y. O., & Adediran, G. O. (1993). The effect of nematicides on the nutritive value and functional properties of cowpea seeds (Vigna unguiculata L. Walp). Food Chemistry, 46, 337–341.
- Oomah, B. D., Corbé, A., & Balasubramanian, P. (2010). Antioxidant and anti-inflammatory activities of bean (Phaseolus vulgaris L.) hulls. Journal of Agricultural and Food Chemistry, 58(14), 8225–8230.
- Orak, H. H., Karamać, M., Orak, A., & Amarowicz, R. (2016). Antioxidant potential and phenolic compounds of some widely consumed turkish white bean (Phaseolus vulgaris L.) varieties. Polish Journal of Food and Nutrition Sciences, 66, 253–260.
- Osorio-Díaz, P., Tovar, J., Paredes‐López, O., Acosta‐Gallegos, J. A., & Bello‐Pérez, L. A. (2005). Chemical composition and in vitro starch bioavailability of Phaseolus vulgaris (L) cv Mayocoba. Journal of the Science of Food and Agriculture, 85(3), 499–504.
- Pedrosa, M. M., Cuadrado, C., Burbano, C., Muzquiz, M., Cabellos, B., Olmedilla-Alonso, B., & Asensio-Vegas, C. (2015). Effects of industrial canning on the proximate composition, bioactive compounds contents and nutritional profile of two Spanish common dry beans (Phaseolus vulgaris L.). Food Chemistry, 166, 68–75.
- Porch, T. G., Cichy, K., Wang, W., Brick, M., Beaver, J. S., Santana-Morant, D., & Grusak, M. A. (2017). Nutritional composition and cooking characteristics of tepary bean (Phaseolus acutifolius Gray) in comparison with common bean (Phaseolus vulgaris L.). Genetic Resources and Crop Evolution, 64(5), 935–953.
- Quiróz-Sodi, M., Mendoza-Díaz, S., Hernández-Sandoval, L., & Carrillo-Ángeles, I. (2018). Characterization of the secondary metabolites in the seeds of nine native bean varieties (Phaseolus vulgaris and P. coccineus) from Querétaro, Mexico. Botanical Sciences, 96, 650–661.
- Ranilla, L. G., Genovese, M. I., & Lajolo, F. M. (2007). Polyphenols and antioxidant capacity of seed coat and cotyledon from Brazilian and Peruvian bean cultivars (Phaseolus vulgaris L.). Journal of Agricultural and Food Chemistry, 55(1), 90–98.
- Rocha-Guzmán, N. E., González-Laredo, R. F., Ibarra-Pérez, F. J., Nava-Berumen, C. A., & Gallegos-Infante, J. A. (2007). Effect of pressure cooking on the antioxidant activity of extracts from three common bean (Phaseolus vulgaris L.) cultivars. Food Chemistry, 100, 31–35.
- Salinas-Moreno, Y., Rojas-Herrera, L., Sosa-Montes, E., & Pérez-Herrera, P. (2005). Composición de antocianinas en variedades de frijol negro (Phaseolus vulgaris L.) cultivadas en México. Agrociencia, 39(4), 385–393.
- Sánchez-Chino, X., Jiménez-Martínez, C., Dávila-Ortiz, G., Álvarez-González, I., & Madrigal-Bujaidar, E. (2015). Nutrient and nonnutrient components of legumes, and its chemopreventive activity: A review. Nutrition and Cancer, 67, 401–410.
- Shao, Y., Hu, Z., Yu, Y., Mou, R., Zhu, Z., & Beta, T. (2018). Phenolic acids, anthocyanins, proanthocyanidins, antioxidant activity, minerals and their correlations in non-pigmented, red, and black rice. Food Chemistry, 239, 733–741.
- Singleton, V. V., Orthofer, R., & Lamuela-Raventos, R. M. (1999). Oxidant and antioxidant (Part A). In L. Packer (Ed.), Methods in enzymology (pp. 299, 152). San Diego, CA: Academic Press.
- Smiricky, M. R., Grieshop, C. M., Albin, D. M., Wubben, J. E., Gabert, V. M., & Fahey, G. C. (2002). The influence of soy oligosaccharides on apparent and true ileal amino acid digestibilities and fecal consistency in growing pigs12. Journal of Animal Science, 80, 2433–2441.
- Sousa-Sánchez, A., & Delgado-Salinas, M., (1993). Biological diversity in México: Origins and Distribution (ed.). (pp. 459). New York: Oxford University Press.
- Strauta, L., Muizniece-Brasava, S., & Alsina, I. (2013). Crude Protein and Ash Content in Different Coloured Phaseolus coccineus L. World Academy of Science, Engineering and Technology, International Journal of Biological, Biomolecular, Agricultural, Food and Biotechnological Engineering, 7(7), 696–701.
- Suárez-Martínez, S. E., Ferriz-Martínez, R. A., Campos-Vega, R., Elton-Puente, J. E., de la Torre Carbot, K., & García-Gasca, T. (2016). Bean seeds: Leading nutraceutical source for human health. CyTA-Journal of Food, 14, 131–137.
- Tucker, M. R. (1999). Essential plant nutrients: Their presence in North Carolina soils and role in plant nutrition (pp. 1–9). Department of Agriculture and Consumer Services, Agronomic Division. Available at: http://cdm16062.contentdm.oclc.org/cdm/ref/collection/p249901coll22/id/461718 (accesed 10 December 2018)
- Vargas-Torres, A., Osorio-Dı́az, P., Islas-Hernández, J. J., Tovar, J., Paredes-López, O., & Bello-Pérez, L. A. (2004). Starch digestibility of five cooked black bean (Phaseolus vulgaris L.) varieties. Journal of Food Composition and Analysis, 17(5), 605–612.
- Viola, A. O., & Anekwe, G. E. (2001). Amino acids and other biochemical components of Ricinus communis (Variety minor), an Anti-conceptive seed. Pakistan Journal of Biological Sciences, 4(7), 866–868.
- Wołosiak, R., Drużyńska, B., Piecyk, M., Majewska, E., & Worobiej, E. (2018). Effect of sterilization process and storage on the antioxidative properties of runner bean. Molecules, 23, 1409.
- Xu, B. J., & Chang, S. K. C. (2007). A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. Journal of Food Science, 72, S159–S166.
- Xu, B. J., Yuan, S. H., & Chang, S. K. C. (2007). Comparative analyses of phenolic composition, antioxidant capacity, and color of cool season legumes and other selected food legumes. Journal of Food Science, 72, S167–S177.
- Xu, Y., & Hanna, M. A. (2011). Nutritional and anti‐nutritional compositions of defatted Nebraska hybrid hazelnut meal. International Journal of Food Science & Technology, 46(10), 2022–2029.
- Zhang, B., Deng, Z., Ramdath, D. D., Tang, Y., Chen, P. X., Liu, R., … Tsao, R. (2015). Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on α-glucosidase and pancreatic lipase. Food Chemistry, 172, 862–872.
- Zhao, Y., Du, S. K., Wang, H., & Cai, M. (2014). In vitro antioxidant activity of extracts from common legumes. Food Chemistry, 152, 462–466.
