ABSTRACT
This study evaluated the effect of Prunus mume polyphenols (PMPs) treatment on qualities and nutrient in winter jujube fruits during storage (4°C). Winter jujube fruits were treated with 5, 10, and 15 mg L−1 PMP, respectively. Compared with the control, PMP treatments effectively preserved the quality and nutrient values of winter jujube fruits. They also enhanced the activities of antioxidant enzymes, inhibited the growth of microorganisms, and delayed postharvest metabolism in the fruits. These findings suggest that PMP, as a green, safe agent, can be potentially developed as a postharvest fruit preservative to substantially extend the shelf life.
RESUMEN
El presente estudio evaluó el efecto del tratamiento con polifenoles de Prunus mume (PMP) en las cualidades y los nutrientes de los frutos de azufaifo de invierno durante su almacenamiento (a 4°C). Para ello, los frutos de azufaifo de invierno fueron tratados con 5, 10 y 15 mg L−1 de PMP. Los resultados permitieron constatar que, en comparación con el control, los tratamientos con PMP preservaron eficazmente los valores de calidad, así como los nutrientes de los frutos de azufaifo de invierno. Además, mejoraron la actividad de las enzimas antioxidantes, inhibieron el crecimiento de microorganismos y retrasaron el metabolismo poscosecha de los frutos. Estos resultados dan cuenta de que, como agente ecológico y seguro, el PMP puede desarrollarse potencialmente como conservante de la fruta después de la cosecha, prolongando sustancialmente su vida útil.
1. Introduction
Winter jujube (Zizyphus jujuba Mill.), a fruit typically eaten fresh. Due to its thin peel, juiciness, special flavor, and abundant nutrients, winter jujube is profoundly favored by the consumers (Zhao et al., Citation2019). However, winter jujube is subject to rapid senescence after harvest, which lead to postharvest decay, dehydration, tissue softening, or flesh browning (L. Zhang et al., Citation2016), resulting in a poor sensory quality and economic loss.
Artificial fruit preservatives are commonly used to maintain the nutritional values and extend the shelf life of postharvest fruits. However, artificial preservatives may be toxic to agricultural products and human bodies (Yan et al., Citation2014). Recently, a number of medicinal herb-derived natural extracts, such as phenolic compounds, flavonoids, and anthocyanins, have been widely applied as non-chemical agents for fruit preservation (Ramzan & Baloch, Citation2019).
Prunus mume Sieb. et Zucc. belongs to Rosaceae family, which is also known as Chinese plum or Japanese apricot. It is cultivated in South China along the Yangtze River (Fang et al., Citation2006; Uematsu et al., Citation1991). Importantly, the fruit of P. mume has good nutrition as well as many medical benefits. For example, it has been used as a traditional Chinese medicine for anthelmintic, antidiarrheal, antipyretic, cough and intestinal disorders (Yan et al., Citation2014). Many studies have shown that P. mume contains rich contents of polyphenolic compounds which have various health benefits, including antibacterial (Valtierra-Rodriguez et al., Citation2010), antioxidant (Khallouki et al., Citation2012), and anti‐inflammatory effects (Shirin et al., Citation2015). In recent years, the plum were found as important sources of phenolic antioxidants and antimicrobial agents (Xia et al., Citation2011). Providing that polyphenols can maintain the nutritional values of fruits during storage, they might be further developed as functional agents for fruit preservation.
To the best of our knowledge, little is known about the influence of P. mume polyphenols (PMPs) on flavor and nutritional values of postharvest fruits. The purpose of this study was to examine the preservative effectiveness of PMPs on winter jujube fruits during storage at 4°C. We assessed the browning, decay, weight loss, respiration intensity, titratable acidity (TA), total soluble solids (TSS), ascorbic acid content, and total phenol content of winter jujube fruits. In addition, several characteristic parameters of fruit senescence, including enzyme activities and microbial growth, were also measured.
2. Materials and methods
2.1. Materials
Winter jujube (Zizyphus jujuba Mill.) cv. Dongzao fruits were harvested from an orchard in Guangzhou, Guangdong, China. The fruits with uniform shape and color and no visible defects were selected.
The chemicals and reagents used in this study were purchased from Qiyun Company (Guangzhou, Guangdong, China).
2.2. PMP preparation
Fresh fruits of P. mume (cv. Baimei) were purchased from Guangdong Bosun Health Food Co., Ltd. (Guangzhou, Guangdong, China) and stored at 4°C. PMP was extracted and purified by X-5 resin according to the method reported in our previous study (Xu et al., Citation2018). Briefly, P. mume was crushed and filtered. Then, the juice passed through the X-5 resin column, and the absorbed polyphenolic compounds on the column were eluted with acidified alcohol aqueous solution. Afterwards, the eluted concentrate was freeze-dried to powder and regarded as the PMP.
Total phenolic content of PMP was measured by the Folin-Cioalteu method (Gomes et al., Citation2020). One gram of PMP was dissolve in 1 L methanol containing 10 ml/L HCl for 20 min at 4°C in darkness. Then, the volumes 0.5 mL of PMP solution and 0.25 mL of the Folin–Ciocalteu reagent was mixed. After 3 min, 2.5 mL of a 20% Na2CO3 solution was added to the reaction mixture. The incubation was performed at room temperature for 1 h. The absorbance of the solutions was measured by spectrophotometer (Genesys 10 UV, Thermo Electron Corporation, Pennsylvania, USA) at a wavelength of 720 nm. A standard curve of gallic acid was used for calculating the total phenolic compounds in PMP and the result was expressed as gram of gallic acid equivalent per gram of PMP (mg GAE/g PMP).
The antioxidant activities of PMP was evaluated with 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonate) (ABTS) and 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) according to previously reported methods (Yang et al., Citation2019). Trolox was used as a positive control, and the results (DPPH and ABTS) were expressed g Trolox equivalents/g PMP.
The individual phenolic composition in PMP was identified by the Agilent 1260 HPLC system (Agilent Technologies, Palo Alto, CA, USA) equipped with DAD detector and autosampler, using a 250 × 4.6 mm, 5 mm Agilent Zorbax SB-C18 column (Palo Alto, CA, USA). The analysis conditions were as follows: column temperature at 30°C; injection volume at 20 μL; HPLC running time of 50 min; injection volume at 10 μL; detection wavelength at 280, 320, and 350 nm. Elution was carried out using 0.4% aqueous solution acetic acid (solution A); and acetonitrile (solution B) with the following gradient: 0–40 min, solution B 5–25%; 40–45 min, solution B 25–35%; 45–50 min, solution B 35–50% (Chen et al., Citation2017).
2.3. PMP treatment on winter jujube fruits
The winter jujube fruits were randomly divided into four groups, with 1440 fruits in each group consisting of three replicates (360 fruits per replicate). The fruits in control group was dipped in deionized water for 5 min at 4°C. The fruits in the other groups were treated with 5, 10, and 15 g/L PMP solutions in 20 L of deionized water for 5 min at 4°C, respectively. After drying with natural air, the fruits were packed into polyethylene bags for storage at 4 ± 0.5°C and 85–90% relative humidity for 15 days. Relevant indices of the fruits were measured every two days during storage.
2.4. Determination of fruit quality
2.4.1. Browning index, weight loss, respiration rate and decay index
Browning index of winter jujube was evaluated based on the scale (0–4) of browned area covering fruit surface (Zhang et al., Citation2015).
Weight loss was calculated as follows (Liu et al., Citation2017): Weight loss (%) = [(W0 − W1)/W0] × 100, where W0 and W1 represent the initial and final fruit weights, respectively.
Fifteen fruits from each replicate of individual treatment were placed in a 2-L glass jar. The jar was sealed for 20-min incubation at 4°C, and a probe was inserted into the jar. Respiration rate was measured by a portable infrared CO2 gas analyzer (GXH-3010E; Huayun Analytical Instrument Co., Ltd., Beijing, China), and expressed as mg/kg/h.
Decay index of the stored fruits was defined as the percentage of fruits with juice leakage, substantial softening, or decay, and expressed as %.
2.4.2. Determination titratable acidity (TA) and total soluble solids (TSS)
Ten fruits from each group were homogenized in 50 mL of distilled water by a blender. TA (using 0.064 as the conversion factor) was calculated by titrating 5 mL of fresh PMP, diluted in 50 mL of deionized water, against 0.1 N NaOH solution. TSS was determined by a pocket refractometer (PAL-1; Atago, Japan).
2.4.3. Ascorbic acid
The content of ascorbic acid in the fruits was measured by 2,6- dichlorophenol indophenol staining (Ramzan & Baloch, Citation2019). Briefly, 5 g fruits from each group was homogenized with 20 mL of metaphosphoric acid (6%) for centrifugation. The supernatant was filtered and titrated against the dye. The ascorbic acid content was expressed as a milligram per 100 g fresh weight (mg/100 g FW).
2.4.4. Total phenolic content
Before the total content measured, the fruit surface was cleaned with deionized water and dried with paper towels to remove excess water in order to avoid the influence of exogenous PMP on the total phenol content. Ten fruits from each group were homogenized in 20 mL of chilled methanol (80%) and then shaken in water bath at 4°C for 30 min. The homogenates were centrifuged at 10,000 × g for 10 min at 4°C. Total phenolic content in the supernatant was measured by the Folin-Cioalteu method (Zhang et al., Citation2013), calculated based on a standard curve generated using gallic acid, and expressed as g/kg FW.
2.4.5. Enzyme activities
One unit of PPO activity was defined as the change of 0.01 in absorbance per minute at 470 nm, and it was expressed as U/g (Zeng et al., Citation2019). Superoxide dismutase (SOD) activity was determined following the method described by Zhao et al. (Citation2020). Peroxidase (POD) activity was determined following the method described by Wang & Chen (Citation2010).
2.4.6. Microbiological evaluations
Ten fruits from each group were homogenized and diluted with sterile 0.1% peptone water, to obtain the microbial count. Serial dilutions were performed in triplicate. Total aerobic mesophilic bacteria were enumerated on Plate Count Agar (PCA; Scharlau Chemie, S.A., Barcelona, Spain) after incubation at 32°C for 2 days. Total yeast and mold were enumerated on potato dextrose agar (PDA; Scharlau Chemie, S.A., Barcelona, Spain) after incubation at 4°C for 2 days. Each enumeration was performed in duplicate and expressed as Log colony-forming units (CFU)/g.
2.5. Statistical analysis
All the data were measured in triplicate and presented as the mean ± standard deviation (SD). Data were subjected to analysis of variance (ANOVA) testing with SPSS17.0 statistical software and comparisons of multiple means were achieved using Duncan’s test at p < .05. The graph presentations were generated in the Origin v2021b (OriginLab Corporation, MA, USA).
3. Results and discussion
3.1. Phenolic contents and antioxidant capacity of PMP
The total phenolic content, antioxidant activity, and individual phenolic composition of PMP are listed in . The total phenolic content was 295.46 ± 2.71 (mg gallic acid equivalent /g), proving that PMP is rich in phenolic compounds. Meanwhile, PMP exhibited intensive antioxidant activities, with 0.52 ± 0.07 g Trolox/g for DPPH assay and 0.47 ± 0.09 g Trolox/g for ABTS assay. Furthermore, neochlorogenic acid was the most abundant phenolic compound, accounting for 36.27% (107.23 ± 1.51 mg/g) of the total phenolic content in PMP, followed by chlorogenic acid (87.36 ± 0.83 mg/g) and cryptochlorogenic acid (61.23 ± 0.67 mg/g). The extremely high content of total phenolic, abundant individual phenolic compounds, and the antioxidant activities observed in PMP collectively suggest that PMP can potentially protect fruits from oxidative damage and spoilage induced by microorganisms.
Table 1. Antioxidant capacities (DPPH and ABTS), total phenolic content, and major individual phenolic compound of PMP. GAE: gallic acid equivalent; Data represent mean values ± standard deviations (n = 3) for each sample.
Tabla 1. Capacidades antioxidantes (DPPH y ABTS), contenido fenólico total y principales compuestos fenólicos individuales de PMP.GAE: equivalente de ácido gálico; los datos representan valores medios ± desviaciones estándar (n = 3) para cada muestra
3.2. Fruit browning, weight loss, and respiration rate
Browning has been considered as a postharvest senescence marker of winter jujube fruits (L. Zhang et al., Citation2016). As depicted in ), winter jujube fruit flesh slightly turned brown during the first 5 days of storage. The browning index in the control group sharply increased and reached 65.77% at the end of storage, while 5, 10, and 15 mg/L PMP treatments reduced the browning index to 59.63, 51.87, and 44.63%, respectively. These results indicate that PMP markedly inhibited the development of browning in winter jujube fruits during storage in a dose dependent manner, with the lowest browning index obtained under 15 mg/L PMP treatment. A previous study has reported a similar finding that apple-derived polyphenols inhibited litchi browning, through reducing the oxidation of phenolic compounds by pro-oxidant enzymes and reinforcing reactive oxygen species (ROS) scavenging system (Su et al., Citation2019).
Figure 1. Browning (a), decay rate (b), weight loss (c), and respiration rate (d) in winter jujube fruits during storage at 4°C.
Figura 1. Pardeamiento (a), tasa de descomposición (b), pérdida de peso (c) y tasa de respiración (d) en frutos de azufaifo de invierno durante el almacenamiento a 4°C
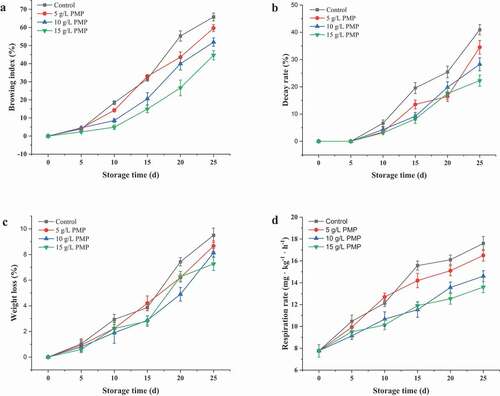
) illustrates that the decay rate of winter jujube fruits in the control group gradually increased after 5 days of storage. At the end of storage, the decay rate of the control was 7.93%, while those of the winter jujube fruits treated with 5, 10, and 15 mg/L PMP were 5.90, 6.87, and 5.07%, respectively, demonstrating that fruit decay was significantly inhibited by PMP. The reduced decay rate might be attributed to the antimicrobial properties of PMP (Wang et al., Citation2019), which plays a crucial role in aerobic bacterial growth inhibition, thus extending the shelf life of fruits.
Fruit weight loss is an important index that reflects respiration rate and moisture evaporation between the fruit tissues and surrounding air (Jiang et al., Citation2020). As shown in ), water loss of winter jujube fruits treated with 15 mg L−1 PMP was the lowest, followed by that treated with 10 mg L−1 ARA, 5 mg L−1, and that of the control was the highest. Water loss was significantly affected by PMP during the storage period (p < .05). During the storage period, retarding the weight loss of winter jujube treated with PMP was better than that of the control. As shown in ), the respiration intensities of control and all treated jujubes were not significantly different at days 0~5; thereafter, respiration intensity in control sharply increased and reached 17.61% at the end of storage, which was significantly higher than the treated group. The respiration intensity of the jujube treated with 15 g/L TPs was the lowest. The results suggest that PMP treatment could inhibit water transpiration while decreasing the cellular respiratory consumption of organic substances of harvested winter jujube fruit, contributing to reduced weight loss and delayed decline in quality of winter jujube fruit.
3.3. Titratable acidity (TA) and total soluble solids (TSS)
shows the changes in TA and TSS throughout the 25-day storage period at 4°C. TA reflects organic acid content changes in fresh fruits during storage. As shown in ), the TA values of winter jujube fruits were decreased by PMP treatments in a time-dependent manner. Generally, fresh fruits are mainly acidic, and the contents of acids usually decrease during ripening when they are utilized for respiration (Martínez〧errer et al., Citation2010). At the 20th day, the TA value of the control decreased to 0.47%, while the values of the treated samples varied from 0.50% to 0.56% ()), indicating the substantial effect of PMP on preserving winter jujube fruits.
Figure 2. Titratable acidity (a) and total soluble solids (b) in winter jujube fruits during storage at 4°C.
Figura 2. Acidez valorable (TA) y sólidos solubles totales (TSS) en frutos de azufaifo de invierno durante el almacenamiento a 4°C
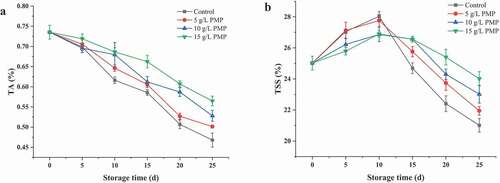
The changes of TSS in winter jujube fruits during storage are presented in ). The TSS values in all the samples slightly increased with fluctuation during the first 10 days of storage, which might be attributed to the breakdown of organic matters for respiration (Moshari-Nasirkandi et al., Citation2020; Shin et al., Citation2007) and the increase of dry matters due to water loss (Silva-Vera et al., Citation2018). Thereafter, the TSS values rapidly decreased with time, which was consistent with our previous findings. The TSS value of the control reached 21.01% at the 11th day, while those of the treated fruits were higher than 21.95%. These results indicate that PMP can slow down fruit metabolic activity, especially respiration, and therefore, maintain the normal level of TSS in fruits.
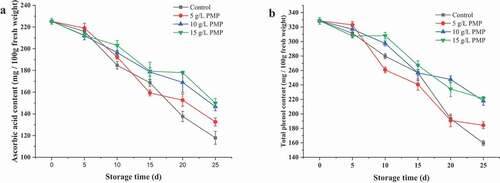
3.4. Ascorbic acid and total phenol contents
shows the changes in the ascorbic acid and total phenol of winter jujube fruits with PMP treated throughout storage. Ascorbic acid and total phenolic content decreased throughout storage ()). The decrease of ascorbic acid and total phenol may be caused by oxidation reaction via oxidase after the harvest (Mditshwa et al., Citation2017). However, there was a more rapid decline in the control than in the fruits treated with PMP (10 and 15 g/L). After day 25, the ascorbic acid and total phenolic content was significantly higher for the fruits with PMP treated in comparison to the controls. This phenomenon suggested the PMP can maintaining the higher ascorbic acid and total phenolic content in comparison to the controls during most of the storage period. The result was in agreement with the report by Su et al. (Citation2019) who demonstrated that the higher concentrations of apple polyphenols could preserve ascorbic acid and total phenol contents in litchi fruits.
3.5. Enzyme activities
Polyphenol oxidase (PPO) activity is closely related to fruit senescence and browning (Cheng et al. Citation2020; H. Zhao et al., Citation2019). PPO-catalyzed oxidative reactions may promote the accumulation of quinones that are further non-enzymatically polymerized into brown melanins, resulting in internal browning in fruit (Tomás-Barberán & Espín, Citation2010). In this study, the PPO activity of winter jujube fruits initially increased and then decreased during storage. At the 10th day of storage, the PPO activity of the control reached the maximum at 21.15 ± 0.17 U/g. In contrast, the PPO activities of the fruits treated with 5, 10, and 15 mg/L PMP were the highest at the 15th day of storage (23.21 ± 0.19, 23.45 ± 0.20, and 23.51 ± 0.31 U/g, respectively), which demonstrates that PMP can enhanced PPO activity in a dose-dependent manner. The groups with PMP treatments were higher control group ()), but the groups with PMP treatments were lower than control group ()). Similar results have been reported by Zeng et al. (Citation2019) that cherry tomato treated with arachidonic acid maintained higher activities of PPO compare with control. Studies have reported that PPO can catalyze oxidative polymerization of phenolic substances to produce quinones, which can inhibit the secretion of pectin and cellulolytic enzyme and improve the fruits defense ability (Zikang et al., Citation2015). Further, PMP and quinones inhibit the secretion of pectin and cellulolytic enzyme and inhibit the Browning of fruits in PMP treated samples. This needs further investigation.
Figure 4. Activities of PPO (a), POD (b), and SOD (c) in winter jujube fruits during storage at 4°C.
Figura 4. Actividades de PPO (a), POD (b) y SOD (c) en frutos de azufaifo de invierno durante el almacenamiento a 4°C
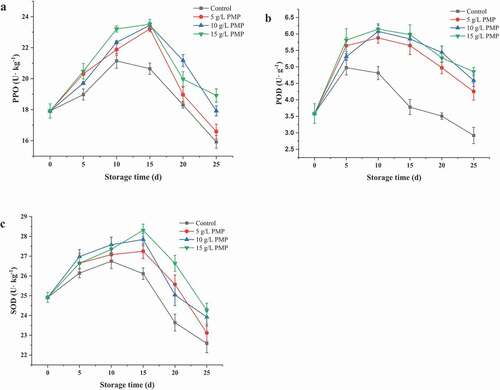
POD is closely related to their physiological and biochemical metabolic processes. As shown in ), the POD activity of winter jujube fruits initially increased and then decreased during storage. Overall, the POD activity of the control, which reached the maximum at the 14th day of storage (4.23 ± 0.22 U/ g), was always lower than those with PMP treatments. In contrast, the POD activities of the fruits treated with 5, 10, and 15 mg/L PMP were the highest at the 15th day of storage (6.53 ± 0.26, 4.86 ± 0.21, and 6.90 ± 0.20 U/g, respectively). These findings indicate that PMP can effectively preserve the activity of POD, which is also supported by X. Zhao et al. (Citation2015), who found that kiwifruit-derived polyphenols effectively increased the POD activity in fresh-cut cantaloupe during storage, thereby delaying senescence and extending the shelf life.
SOD is an important active oxygen scavenging enzyme that prevents plants from oxidative damage and senescence (Jang & Moon, Citation2011). As shown in ), the SOD activity of winter jujube fruits initially increased and then decreased during storage. The SOD activity of the control peaked at the 7th day of storage (231.02 ± 5.32 U/g), while those treated with 5, 10, and 15 mg/L PMP peaked at the 15th day of storage (42.03 ± 1.45, 38.05 ± 1.40, and 48.52 ± 1.40 U/g, respectively). From the 14th to the 42nd day of storage, although the SOD activities of the treatment groups were decreasing, they were always significantly higher than that of the control (P < .05). Similarly, as reported by Su et al. (Citation2019), apple-derived polyphenols effectively increased the activities of POD and SOD in litchi fruits, delaying senescence, and extending the shelf life.
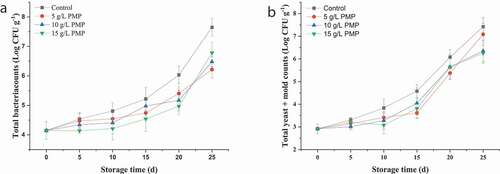
3.6. Bacterial content
Microorganisms are important factors for fruit spoilage during storage. The total bacterial and yeast/mold counts in winter jujube fruits were determined during storage ()). The initial populations of total bacteria and yeast/mold in the winter jujube fruits were 4.15 ± 0.30 and 2.92 ± 0.20 log CFU g−1, respectively, and increased during fruit storage in MPM treated and control, but it was greater in untreated fruit than those of the PMP treatments. At the end of storage, the total bacterial count of the control was 7.65 log CFU/g, while those of winter jujube fruits treated with 5, 10, and 15 mg/L PMP were 6.22, 6.48, and 6.78 log CFU/g, respectively; the yeast/mold content of the control was 7.45 log CFU/g, while those of winter jujube fruits treated with 5, 10, and 15 mg/L PMP were 7.08, 6.35, and 6.25 log CFU/g. Polyphenols are plant secondary metabolites having strong antibacterial activity, have been utilized in food preservation. It was reported by Xia et al. (Citation2011) that P. mume-derived polyphenols had a strong inhibitory effect on microorganisms. Therefore, PMP can delay fruit senescence and extend the shelf life by inhibiting the growth of microorganisms.
4. Conclusion
The PMP isolated from P. mume fruit mainly composed of neochlorogenic acid, cryptochlorogenic acid, chlorogenic acid and caffeic acid that was displayed strong antioxidant activity. It effectively preserved the quality and nutrient values of winter jujube fruits. Meanwhile, PMP treatment also enhanced the activities of antioxidant enzymes, inhibited the growth of microorganisms, and delayed postharvest metabolism in the fruits. Therefore, PMP is a promising agent for maintaining the sensory and nutritional attributes of postharvest fruits, and it can be used as an effective natural fruit preservative.
Author contributions
Yangyang Yu and Jing Wen collected the data and wrote the manuscript. Yuanshan Yu and Yujuan Xu designed the experiments, analyzed the data and reviewed the manuscript. All of the authors completed and authorized the definitive manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Chen, Z. Y., Lin, Y. S., Liu, X. M., Cheng, J. R., & Yang, C. Y. (2017). Chemical composition and antioxidant activities of five samples of prunus mume umezu from different factories in South and East China. Journal of Food Quality, 2017, 1–7. https://doi.org/https://doi.org/10.1155/2017/4878926
- Cheng, S., Yu, Y., Guo, J., Chen, G., & Guo, M. (2020). Effect of 1-methylcyclopropene and chitosan treatment on the storage quality of jujube fruit and its related enzyme activities. Scientia Horticulturae, 265, 109–114. https://doi.org/https://doi.org/10.1016/j.scienta.2020.109281
- Fang, J., Twito, T., Zhen, Z., & Chao, C. (2006). Genetic relationships among fruiting-mei (Prunus mume Sieb. et Zucc.) cultivars evaluated with AFLP and SNP markers. Genome, 49(10), 1256–1264. https://doi.org/https://doi.org/10.1139/g06-097
- Gomes, A. C. A., Da Costa Lima, M., De Oliveira, K. A. R., Dos Santos Lima, M., Magnani, M., Camara, M. P. S., & De Souza, E. L. (2020). Coatings with chitosan and phenolic-rich extract from acerola (Malpighia emarginata D.C.) or jabuticaba (Plinia jaboticaba (Vell.) Berg) processing by-product to control rot caused by Lasiodiplodia spp. in papaya (Carica papaya L.) fruit. International Journal of Food Microbiology, 331, 1086–1094. https://doi.org/https://doi.org/10.1016/j.ijfoodmicro.2020.108694
- Jang, J. H., & Moon, K. D. (2011). Inhibition of polyphenol oxidase and peroxidase activities on fresh-cut apple by simultaneous treatment of ultrasound and ascorbic acid. Food Chemistry, 124(2), 444–449. https://doi.org/https://doi.org/10.1016/j.foodchem.2010.06.052
- Jiang, Y., Yu, L., Hu, Y., Zhu, Z., & Zhong, Y. (2020). The preservation performance of chitosan coating with different molecular weight on strawberry using electrostatic spraying technique. International Journal of Biological Macromolecules, 21, 151–162. https://doi.org/https://doi.org/10.1016/j.ijbiomac.2020.02.169
- Khallouki, F., Haubner, R., Erben, G., Ulrich, C. M., & Owen, R. W. (2012). Phytochemical composition and antioxidant capacity of various botanical parts of the fruits of Prunus × domestica L. from the Lorraine region of Europe. Food Chemistry, 133(3), 697–706. https://doi.org/https://doi.org/10.1016/j.foodchem.2012.01.071
- Liu, Y., Wang, S., Lan, W., & Qin, W. (2017). Fabrication and Testing of PVA/Chitosan bilayer films for strawberry packaging. Coatings, 7(8), 109–125. https://doi.org/https://doi.org/10.3390/coatings7080109
- Martínez〧errer, M., Harper, C., Pérez㎝untoz, F., & Chaparro, M. (2010). Modified atmosphere packaging of minimally processed mango and pineapple fruits. Journal of Food Science, 67(9), 3365–3371. https://doi.org/https://doi.org/10.1111/j.1365-2621.2002.tb09592.x
- Mditshwa, A., Magwaza, L. S., Tesfay, S. Z., & Opara, U. L. (2017). Postharvest factors affecting vitamin C content of citrus fruits: A review. Scientia Horticulturae, 218, 95–104. https://doi.org/https://doi.org/10.1016/j.scienta.2017.02.024
- Moshari-Nasirkandi, A., Alirezalu, A., & Hachesu, M. A. (2020). Effect of lemon verbena bio-extract on phytochemical and antioxidant capacity of strawberry (Fragaria×ananassa Duch. cv. Sabrina) fruit during cold storage. Biocatalysis and Agricultural Biotechnology, 25, 1016–1023. https://doi.org/https://doi.org/10.1016/j.bcab.2020.101613
- Ramzan, M., & Baloch, A. H. (2019). Effect of Aloe vera gel coating enriched with Fagonia indica plant extract on physicochemical and antioxidant activity of sapodilla fruit during postharvest storage. Food Chemistry, 286, 346–353. https://doi.org/https://doi.org/10.1016/j.foodchem.2019.01.135
- Shin, Y., Liu, R. H., Nock, J. F., Holliday, D., & Watkins, C. B. (2007). Temperature and relative humidity effects on quality, total ascorbic acid, phenolics and flavonoid concentrations, and antioxidant activity of strawberry. Postharvest Biology and Technology, 45(3), 349–357. https://doi.org/https://doi.org/10.1016/j.postharvbio.2007.03.007
- Shirin, H., Ajay, K., Ji, Y., Zhang, S., & Johnson. (2015). Evidence for anti-inflammatory and antioxidative properties of dried plum polyphenols in macrophage RAW 264.7 cells. Food & Function, 14, 342–351. https://doi.org/https://doi.org/10.1039/c5fo00173k
- Silva-Vera, W., Zamorano-Riquelme, M., Rocco-Orellana, C., Vega-Viveros, R., Gimenez-Castillo, B., Silva-Weiss, A., & Osorio-Lira, F. (2018). Study of spray system applications of edible coating suspensions based on hydrocolloids containing cellulose nanofibers on grape surface (Vitis vinifera L.). Food and Bioprocess Technology, 11(8), 1575–1585. https://doi.org/https://doi.org/10.1007/s11947-018-2126-1
- Su, Z., Hu, M., Gao, Z., Li, M., & Jiang, Y. (2019). Apple polyphenols delay senescence and maintain edible quality in litchi fruit during storage. Postharvest Biology and Technology, 157, 976–981. https://doi.org/https://doi.org/10.1016/j.postharvbio.2019.110976
- Tomás-Barberán, F., & Espín, J. (2010). Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. Journal of the Science of Food and Agriculture, 81(9), 853–876. https://doi.org/https://doi.org/10.1002/jsfa.885
- Uematsu, C., Sasakuma, T., & Ogihara, Y. (1991). Phylogenetic relationships in the stone fruit group of Prunus as revealed by restriction fragment analysis of chloroplast DNA. Idengaku Zasshi, 66(1), 59–69. https://doi.org/https://doi.org/10.1266/jjg.66.59
- Valtierra-Rodriguez, D., Heredia, N. L., Garcia, S., & Sanchez, E. (2010). Reduction of campylobacter jejuni and campylobacter coli in poultry skin by fruit extracts. Journal of Food Protection, 73(3), 477–482. https://doi.org/https://doi.org/10.4315/0362-028X-73.3.477
- Wang, S. Y., & Chen, C. T. (2010). Effect of allyl isothiocyanate on antioxidant enzyme activities, flavonoids and post-harvest fruit quality of blueberries (Vaccinium corymbosum L., cv. Duke). Food Chemistry, 122(4), 1153–1158. https://doi.org/https://doi.org/10.1016/j.foodchem.2010.03.106
- Wang, X., Du, J., & Zhou, J. (2019). Antibiotic activities of extracts from Prunus mume fruit against food-borne bacterial pathogens and its active components. Industrial Crops and Products, 133, 409–413. https://doi.org/https://doi.org/10.1016/j.indcrop.2019.02.050
- Xia, D., Wu, X., Shi, J., Yang, Q., & Zhang, Y. (2011). Phenolic compounds from the edible seeds extract of Chinese Mei (Prunus mume Sieb. et Zucc) and their antimicrobial activity. LWT - Food Science and Technology, 44(1), 347–349. https://doi.org/https://doi.org/10.1016/j.lwt.2010.05.017
- Xu, L., Zhu, M. J., Liu, X. M., & Cheng, J. R. (2018). Inhibitory effect of mulberry (Morus alba) polyphenol on the lipid and protein oxidation of dried minced pork slices during heat processing and storage. LWT - Food Science and Technology, 91, 222–228. https://doi.org/https://doi.org/10.1016/j.lwt.2018.01.040
- Yan, X. T., Lee, S. H., Li, W., Sun, Y. N., Yang, S. Y., Jang, H. D., & Kim, Y. H. (2014). Evaluation of the antioxidant and anti-osteoporosis activities of chemical constituents of the fruits of Prunus mume. Food Chemistry, 156(aug.1), 408–415. https://doi.org/https://doi.org/10.1016/j.foodchem.2014.01.078
- Yang, J., Siew, Y., Quek, M., Gu, Y., & Guo, Y. (2019). Polyphenols from thinned young kiwifruit as natural antioxidant: Protective effects on beef oxidation, physicochemical and sensory properties during storage - ScienceDirect. Food Control, 108, 1068–1078. https://doi.org/https://doi.org/10.1016/j.foodcont.2019.106870
- Zeng, C., Tan, P., & Liu, Z. (2019). Effect of exogenous ARA treatment for improving postharvest quality in cherry tomato (Solanum lycopersicum L.) fruits. Scientia Horticulturae, 261, 108959. https://doi.org/https://doi.org/10.1016/j.scienta.2019.108959
- Zhang, L., Li, S., Dong, Y., Zhi, H., & Zong, W. (2016). Tea polyphenols incorporated into alginate-based edible coating for quality maintenance of Chinese winter jujube under ambient temperature. LWT - Food Science and Technology, 70, 155–161. https://doi.org/https://doi.org/10.1016/j.lwt.2016.02.046
- Zhang, Z., Huber, D. J., Qu, H., Yun, Z., Wang, H., Huang, Z., Huang, H., & Jiang, Y. (2015). Enzymatic browning and antioxidant activities in harvested litchi fruit as influenced by apple polyphenols. Food Chemistry, 171(mar.15), 191–199. https://doi.org/https://doi.org/10.1016/j.foodchem.2014.09.001
- Zhang, Z., Huber, D. J., & Rao, J. (2013). Antioxidant systems of ripening avocado (Persea americana Mill.) fruit following treatment at the preclimacteric stage with aqueous 1-methylcyclopropene. Postharvest Biology and Technology, 76(24), 438–249. https://doi.org/https://doi.org/10.1016/j.postharvbio.2012.09.003
- Zhao, H., Liu, B., Zhang, W., Cao, J., & Jiang, W. (2019). Enhancement of quality and antioxidant metabolism of sweet cherry fruit by near-freezing temperature storage. Postharvest Biology and Technology, 147, 113–122. https://doi.org/https://doi.org/10.1016/j.postharvbio.2018.09.013
- Zhao, X., Li, L., Luo, Q., Ye, M., Luo, G., & Kuang, Z. (2015). Effects of mulberry (Morus alba L.) leaf polysaccharides on growth performance, diarrhea, blood parameters, and gut microbiota of early-weanling pigs. Livestock Science, 177, 88–94. https://doi.org/https://doi.org/10.1016/j.livsci.2015.03.001
- Zhao, Y., Zhu, X., Hou, Y., Wang, X., & Li, X. (2019). Effects of nitric oxide fumigation treatment on retarding cell wall degradation and delaying softening of winter jujube (Ziziphus jujuba Mill. cv. Dongzao) fruit during storage. Postharvest Biology and Technology, 156, 1109–1124. https://doi.org/https://doi.org/10.1016/j.postharvbio.2019.110954
- Zhao, Y., Zhu, X., Hou, Y., Wang, X., & Li, X. (2020). Postharvest nitric oxide treatment delays the senescence of winter jujube (Zizyphus jujuba Mill. cv. Dongzao) fruit during cold storage by regulating reactive oxygen species metabolism. Scientia Horticulturae, 261, 342–353. https://doi.org/https://doi.org/10.1016/j.scienta.2019.109009
- Zikang, C., Rong, Y., Hongmei, X., Xiaojie, Q., & Linyuan, S. (2015). Effect of preharvest application of Hanseniaspora uvarum on postharvest diseases in strawberries - ScienceDirect. Postharvest Biology and Technology, 100(100), 52–58. https://doi.org/https://doi.org/10.1016/j.postharvbio.2014.09.004