ABSTRACT
Glucosamine (GlcN) has been widely used to treat osteoarthritis. However, GlcN derived from crustacean shells has several drawbacks, and it is not vegan-friendly. Here, we produced GlcN from the biomass of Aspergillus oryzae NCH-42 and investigated the effects of environmental factors (e.g. carbon and nitrogen sources, temperature, and pH) on the GlcN production of the fungal biomass. The acidic stress resulted in significantly higher GlcN content in the cell wall of A. oryzae NCH-42. Moreover, the SEM examination revealed that the mycelial structure grown at pH 2.5 was thick and sturdy, compared to that at neutral pH conditions. The final yield of GlcN could be enhanced by up to 7.75-fold by cultivating at modified PDB with an initial pH of 2.5 at 30°C for 4 days. In this study, the GlcN content of A. oryzae biomass could reach up to 0.31 g/g biomass, indicating its high potentiality for GlcN production.
La glucosamina (GlcN) se ha utilizado ampliamente para tratar la artrosis. Sin embargo, aquella que deriva de los caparazones de crustáceos presenta varios inconvenientes y no es apta para veganos. En el presente estudio produjimos GlcN a partir de la biomasa de Aspergillus oryzae NCH-42 e investigamos los efectos de los factores ambientales (por ejemplo, las fuentes de carbono y nitrógeno, la temperatura y el pH) en la producción de GlcN de la biomasa fúngica. El estrés ácido dio lugar a un contenido de GlcN significativamente mayor en la pared celular de A. oryzae NCH-42. Además, el examen SEM permitió comprobar que la estructura micelial cultivada a pH 2.5 era gruesa y robusta, en comparación con la cultivada en condiciones de pH neutro. El rendimiento final de GlcN se pudo multiplicar hasta por 7.75 al cultivarla en PDB (caldo de dextrosa de papa [patata]) modificado con un pH inicial de 2.5 a 30°C durante 4 días. En este estudio, el contenido de GlcN de la biomasa de A. oryzae alcanzó hasta 0.31 g/g de biomasa, lo que indica su alta potencialidad para producir GlcN.
1. Introduction
Glucosamine (also known as 2-amino-2-deoxy-D-glucose, GlcN), an amino monosaccharide, is one of the structural units of chitin and chitosan. It is widely used as an over-the-counter supplement to treat osteoarthritis. Numerous studies have also indicated that GlcN exhibited various therapeutic effects, including anti-oxidation, anti-inflammation, anti-cancer, anti-microbial activity, anti-aging, atheroprotection, cardioprotection, chondroprotection, immunomodulation, and neuroprotection (Dalirfardouei et al., Citation2016). Among the biomedical effects reported, the anti-inflammatory and chondroprotective efficacies of GlcN have evidently been shown in the treatment of osteoarthritis (Lee et al., Citation2010; McAlindon et al., Citation2000).
Most of the commercial glucosamine products are produced by the hydrolysis of chitin from crustaceans (especially shrimp and crab) with chemicals (e.g. concentrated hydrochloric acid) or enzymes (e.g. endochitinase, exochitinase, chitobiase, and β-N-acetylhexosaminidase) (Liu et al., Citation2013; Nidheesh et al., Citation2015; Shahidi et al., Citation1999). However, the production of GlcN from crustaceans is subject to certain limitations, including seasonal variations and limited supply of raw materials, high costs, laborious process, not eco-friendly, and protein contamination (Dhillon et al., Citation2012). Besides that, the crustaceans-derived GlcN is not suitable for vegetarians and people who are allergic to crustaceans. Hence, more efforts have been concentrated on the research of GlcN production from fungal sources (Peng & Wu, Citation2020; Wang et al., Citation2014).
GlcN obtained from fungal cell wall is an alternative source. Fungal GlcN is relatively inexpensive, productive, and environmentally friendly (Ghormade et al., Citation2017; Sitanggang et al., Citation2012). Previous studies have identified Rhizopus oligosporus (Hsieh et al., Citation2007; Sparringa & Owens, Citation1999), Aspergillus sp. (Carter et al., Citation2004; Chang et al., Citation2011), Rhizopus oryzae (Liao et al., Citation2008) as potential fungal strains for GlcN production. These studies have evidently shown the high potentiality of the biological production of GlcN and are worth further exploration. To the best of our knowledge, none of these studies have made an attempt on GlcN production from the biomass of Aspergillus oryzae, which is a type of filamentous fungus first isolated from koji and generally recognised as safe (GRAS). This strain has been widely used in the production of fermented food products, such as sake (Japanese rice wine), miso, and soy sauce (Sparringa & Owens, Citation1999).
The fungal cell wall is a dynamic structure that maintains cellular shape and integrity as well as withstands environmental stresses. Several reports have pointed out that the cell wall architecture and composition of filamentous fungi could be affected by the nutrients obtained and environmental factors, such as pH and temperature (Te Biesebeke et al., Citation2005; Latgé, Citation2010; Yoshimi et al., Citation2017). As a vital constituent of the fungal cell wall, chitin plays an important role in providing cross-linking strength to the fungal cell wall, and its amount varies in response to the variations in supplemented nutrients and environmental stresses, like hypoxia, change of carbon sources, and immune attack (Hopke et al., Citation2018). This would eventually affect the production yield of GlcN (the basic composing amino sugar unit of chitin) (Sitanggang et al., Citation2012). Several reports have been published on chitin and the chitosan content of Aspergillus niger; nevertheless, scarce reports on the investigation of A. oryzae were found, not to mention its GlcN content. To the best of our knowledge, the effects of culturing conditions for A. oryzae, especially acidic stress on the mycelial growth and biomass concentration, have rarely been investigated. Therefore, understanding these parameters could be the key to significant changes in the cell wall structure, thus enhancing the yield of GlcN.
Potato dextrose broth (PDB) is considered as a standard medium for the activation and maintenance of fungal cultures. It comprised potato starch infusion (4 g/L medium) and dextrose (20 g/L medium). This may not supply sufficient nutrients for fungi to effectively generate large amount of biomass for GlcN extraction. Hence, the supplementation of additional carbon and nitrogen sources is required. In this study, the effects of physical and chemical factors, including the initial pH value, culturing temperature, carbon source, and nitrogen source, on the biomass and GlcN production of A. oryzae NCH-42 were investigated. The changes in the cellular surface were also compared with and without the acidic stress through scanning electron microscopy to confirm the close relation between hyphal cell wall structure and GlcN content.
2. Materials and methods
2.1. Chemicals
3,5-Dinitrosalicylic acid (DNS), D-fructose, glucosamine hydrochloride, D-mannose, 1-naphthyl isothiocyanate, and pyridine were purchased from Sigma-Aldrich (USA). D-glucose, polyoxyethylene sorbitan monooleate (Tween 80), and urea were obtained from Hayashi Pure Chemical Ind. Ltd. (Japan). Ammonium sulfate, hydrochloric acid (HCl), and sodium hydroxide (NaOH) were purchased from Union Chemical Works Ltd. (Taiwan). Acetonitrile was obtained from Merck (Germany). Maltose, potato dextrose broth (PDB), potato dextrose agar (PDA), peptone, and yeast extract were obtained from Difco Co. (USA). Ethanol and ammonium nitrate were purchased from Shen Chiu Enterprise (Taiwan) and Katayama Chemical Industries Co., Ltd. (Japan), respectively.
2.2. Microorganism
A. oryzae NCH-42 was isolated from the soil collected at the mountains of Taiwan and identified by Bioresources Collection and Research Center of the Food Industry Research and Development Institute (Taiwan). The strain was cultivated on PDA at 30°C until sporulation was completed (3–5 days). The spores were harvested by adding sterile 0.1% Tween 80 solution to the cultures and scrapped with a sterile inoculating loop. Subsequently, the spore suspension was filtered through gauze. Concentration of spore suspension was microscopically determined using a hemacytometer (Neubauer improved cell counting chamber, Electron Microscopy Sciences, USA) and adjusted with sterile water to approximately 108 spores/mL.
2.3. Evaluation of culture conditions for biomass concentration and GlcN production
The spore suspension of A. oryzae NCH-42 (5 mL) was inoculated into a 250 mL Erlenmeyer flask containing 50 mL of the medium. Then, the inoculated medium was incubated at 30°C for 7 days in an orbital shaker set at 200 rpm. The basal medium used in this study was PDB.
2.3.1. Effect of carbon source
In order to evaluate the effect of various carbon sources on the GlcN production, 20 g/L of fructose, glucose, maltose, and mannose were, respectively, supplemented in the basal medium. The basal medium was used as the control in this section of the study.
2.3.2. Effect of nitrogen source
Different nitrogen sources, including peptone, yeast extract, ammonium sulfate, ammonium nitrate, and urea (with nitrogen equivalent adjusted to 2.86 g/L medium) (), were added, respectively, to the basal medium supplemented with 20 g/L of glucose to investigate their effects on mycelial growth and the production of GlcN. Subsequently, different carbon-to-nitrogen ratios (C/N ratio of 1.63–4.89:1) were evaluated for maximal biomass concentration and GlcN production (). The control of this section of the study is the basal medium supplemented with 20 g/L of glucose.
2.3.3. Effect of culturing pH and temperature
After investigating the effect of carbon and nitrogen sources on the mycelial growth and GlcN production, the modified medium (also known as modified PDB) was used at this part of the study. A. oryzae NCH-42 was cultured at the modified medium with different initial pH (2, 2.5, 3, 3.5, 4, 5, 6, and 7) and temperatures (20°C, 25°C, 30°C, 35°C, and 40°C) for the maximal biomass concentration and GlcN production by using one variable at a time approach in a submerged fermentation. The pH of the medium was adjusted with 6 N HCl or 5 N NaOH.
2.4. Determination of biomass dry weight
The total biomass obtained after cultivation in the media of each flask was collected and filtered with pre-weighed filter paper (Whatman No. 1), followed by washing with reversed osmosis water to remove the medium. The biomass together with the filter paper was dried in an oven at 60°C. The total weight of biomass together with filter paper was then measured up to a constant weight. The dry weight of the biomass was calculated by subtracting the weight of filter paper from the total weight.
2.5. Determination of residual sugar in the culture broth
The residual sugar concentration was determined according to the methods of Miller (Citation1959) with some modifications. Sample aliquots (100 μL) were mixed with 300 μL DNS and reacted at 100°C for 5 min. After rapid cooling, 500 μL distilled water was added into the mixture, and its absorbance was measured at 540 nm using a multi-mode microplate reader (FLUO Star Omega, BMG Labtech, Germany). The residual sugar concentration was calculated based on its corresponding standard curve of glucose and expressed as gram of glucose equivalent per litre of media.
2.6. Extraction and determination of GlcN concentration from fungal biomass
The determination of GlcN concentration was in accordance with the method reported by Sitanggang et al. (Citation2009) with some modifications. One gram of dried biomass was hydrolyzed with 10 mL of 6 N HCl at 100°C for 4 h. The hydrolyzed biomass was cooled to ambient temperature and 10 mL of deionized water was added. After that, the biomass was neutralized to pH 7 with 5 N NaOH and filtered through Whatman No. 1 filter paper. The biomass solution was stored at −20°C for subsequent analysis.
A 0.2 mL aliquot of the above biomass solution was mixed with 0.6 mL 0.04 M 1-naphthyl isothiocyanate pyridine and reacted at 50°C for 1 h. After passing the mixture through a 0.22 μm filter, 10 μL aliquot was withdrawn and analyzed by HPLC (Hitachi Ltd., Japan) with a reverse phase column (Mightysil RP-18 GP 250 × 4.6 mm, 5 μm, Kanto Corporation, USA) at a flow rate of 1.3 mL/min for 40 min. Acetonitrile (87%, v/v) diluted in deionized water was used as the mobile phase, while the ultraviolet wavelength was adjusted to 230 nm. The GlcN concentration of the sample was calculated using the following equation:
GlcN concentration (g/L medium) = biomass concentration (g/L medium) × GlcN content (g/g biomass)
2.7. Scanning electron microscopy (SEM)
In order to observe the morphological differences on the surface of A. oryzae NCH-42, a field-emission scanning electron microscope (JSM-7800 F Prime, JEOL, Japan) was used. The biomass collected was dehydrated stepwise with 25%, 50%, 75%, and 100% alcohol solution after rinsing with reversed osmosis water. Finally, the biomass was freeze-dried to remove the residual moisture and fixed on aluminium stub with double conductive copper foil tape. The treated sample was then sputtered with platinum and observed under vacuum condition. The fungal cells that were cultivated in the modified PDB with (pH 2.5) and without (pH 6.5) pH adjustment were used as the samples.
2.8. Statistical analysis
Data were reported in mean ± standard deviation. Statistical differences were carried out with one-way analysis of variance (ANOVA) and Scheffé’s method using Statistical Product and Service Solutions (SPSS, version 20.0, IBM Inc., USA). Values of p< .05 were considered to be statistically significant.
3. Results and discussion
3.1. Effect of carbon source
Carbon source has been reported to be an important factor in the growth of filamentous fungi (Hamad et al., Citation2014; Jun et al., Citation2013). demonstrates the effect of different supplemented carbon sources (including fructose, glucose, maltose, and mannose) on the biomass concentration () and GlcN production () of A. oryzae NCH-42. It was observed that the supplementation of carbon source resulted in a significantly (p< .05) higher (75–83%) biomass concentration. However, different carbon sources did not have obvious differences in the biomass concentration (13.07–13.71 g/L medium).
Figure 1. Variations in (a) biomass concentration and (b) glucosamine (GlcN) production of A. oryzae NCH-42 at potato dextrose broth (PDB) supplemented with different carbon sources. PDB alone was used as the medium for the control group. PDB supplemented with different carbon sources (20 g/L medium) were used as the media for the experimental groups. Bars with different letters are significantly (p < .05) different.
Figura 1. Variaciones en (a) la concentración de biomasa y (b) la producción de glucosamina (GlcN) de A. oryzae NCH-42 en caldo de dextrosa de papa (PDB) suplementado con diferentes fuentes de carbono. El PDB se utilizó como medio solo para el grupo de control. El PDB complementado con diferentes fuentes de carbono (20 g/L de medio) se utilizó como medio para los grupos experimentales. Las barras con letras distintas son significativamente (p < .05) diferentes
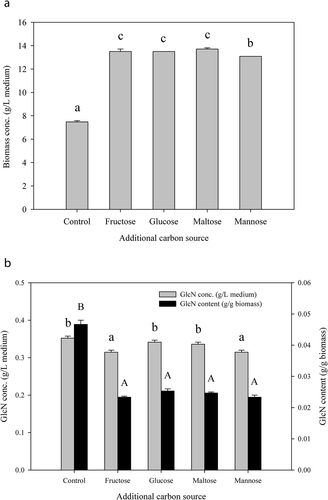
As shown in , all of the experimental groups had lower GlcN content (0.02–0.03 g/g biomass) than the control group (0.05 g/g biomass). The GlcN concentration cultivated on the media supplemented with glucose (0.34 g/L medium) and maltose (0.34 g/L medium) was comparable to the control group (0.35 g/L medium), while GlcN concentration grown on the media incorporated with fructose (0.31 g/L medium) and mannose (0.31 g/L medium) was both lower than the control group. Although the results indicated that the carbon source used in this study could be a fundamental element to enhance the biomass concentration of A. oryzae NCH-42, it could not enhance the production of GlcN.
According to the report by Gao et al. (Citation2007), carbon source and C/N ratio are factors that affect the fungal growth and sporulation. Therefore, it is necessary to evaluate the effect of C/N ratio on the mycelial growth and GlcN production. According to Ene et al. (Citation2012), the carbon source in the medium could affect the architecture and biophysical properties of fungal cell wall. Despite the higher growth rate (or biomass concentration) obtained with the supplementation of carbon source in the media, the presence of a particular carbon source could result in a reduction of chitin/glucan layer while increasing the mannan layer of the cell wall (Ene et al., Citation2012). Moreover, glucose is the key compound that underwent several reactions to form uridine diphosphate (UDP) N-acetyl glucosamine and further to chitin via chitin synthase (Elsoud & El Kady, Citation2019; Nwe et al., Citation2011). Based on the chitin synthesis pathway and the result of carbon source, glucose was therefore selected as the carbon source for GlcN production.
3.2. Effect of nitrogen source
Nitrogen source is an important nutrient for the growth and activity of fungus (Di Lonardo et al., Citation2020). Particularly, nitrogen sources are used as substrates by Aspergillus genus during metabolism through the pathway of nitrate assimilation (Krappmann & Braus, Citation2005). As shown in ), the supplementation of different nitrogen sources did not enhance the biomass concentration of A. oryzae NCH-42. The addition of yeast extract to PDB showed comparable biomass concentration (12.55 g/L medium) to the control group (13.09 g/L medium), while the inclusion of ammonium sulfate (10.36 g/L medium), peptone (8.00 g/L medium), ammonium nitrate (4.91 g/L medium), and urea (4.36 g/L medium) resulted in a lower biomass concentration.
Figure 2. Variations in (a) biomass concentration and (b) glucosamine (GlcN) production of A. oryzae NCH-42 at potato dextrose broth (PDB) supplemented with different nitrogen sources. PDB supplemented solely with glucose (20 g/L medium) was used as the medium for the control group. PDB supplemented with glucose (20 g/L medium) and nitrogen (equivalent to 2.86 g/L medium) were used as the media for the experimental groups. Bars with different letters are significantly (p < .05) different.
Figura 2. Variaciones en (a) la concentración de biomasa y (b) la producción de glucosamina (GlcN) de A. oryzae NCH-42 en caldo de dextrosa de papa (PDB) suplementado con diferentes fuentes de nitrógeno. El PDB suplementado únicamente con glucosa (20 g/L de medio) se utilizó como medio para el grupo de control. El PDB suplementado con glucosa (20 g/L de medio) y nitrógeno (equivalente a 2.86 g/L de medio) se utilizó como medio para los grupos experimentales. Las barras con letras distintas son significativamente (p < .05) diferentes
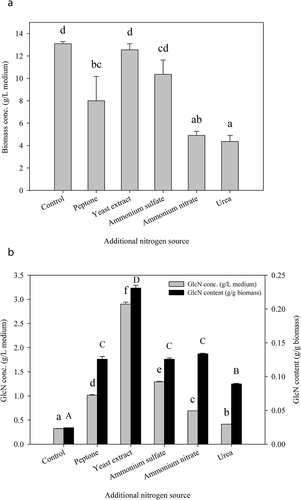
) reveals that the GlcN concentrations of the control, peptone, yeast extract, ammonium sulfate, ammonium nitrate, and urea groups were 0.32, 1.01, 2.90, 1.29, 0.69, and 0.41 g/L medium, respectively. Despite the fact that additional nitrogen source might lower the biomass concentration, it was noted that the supply of additional nitrogen source in this study significantly improved the GlcN concentration of A. oryzae NCH-42 by 29–800%. The highest GlcN content produced by A. oryzae NCH-42 was found with PDB supplemented with yeast extract (0.23 g/g biomass). As expected, the control group in this study produced both the lowest GlcN concentration (0.32 g/L medium) and GlcN content (0.02 g/g biomass). Among the supplemented nitrogen sources, yeast extract exhibited the best nitrogen source for GlcN production by A. oryzae NCH-42. Chhabra et al. (Citation2014) also showed that the supplementation of media with yeast extract is a promising way to improve the production of fungal chitosan, the deacetylated form of chitin structure.
To determine the appropriate amount of yeast extract as the nitrogen source for A. oryzae NCH-42, various C/N ratios (1.63 to 4.89:1) on the GlcN production of A. oryzae NCH-42 biomass were investigated. The results in indicated that the lower the C/N ratio (the higher the concentration of yeast extract), the higher the biomass concentration. However, GlcN content of A. oryzae NCH-42 appeared to be the most efficient at C/N ratio of 2.45 to 3.67. The highest biomass and GlcN concentrations at that particular range were found at C/N ratio of 2.45.
Table 1. Variations in the production of glucosamine (GlcN) by A. oryzae NCH-42 with different carbon-to-nitrogen ratio (C/N ratio).
Tabla 1. Variaciones en la producción de glucosamina (GlcN) por A. oryzae NCH-42 con diferentes ratios carbono-nitrógeno (ratio C/N))
3.3. Effect of temperature
A. oryzae could be grown at a wide range of temperatures, which ranged from 20°C to 40°C (Sakurai et al., Citation1985). Therefore, the effect of temperature on the production of GlcN from A. oryzae NCH-42 was investigated under this range of temperature (). The biomass concentration produced by A. oryzae NCH-42 (18.60 g/L medium) in this study was found to achieve the highest at 20°C. Nonetheless, the GlcN concentration and GlcN content appeared to be less productive at 20°C, which were 2.25 g/L medium and 0.12 g/g biomass, respectively. The GlcN production had higher efficiency at 25°C and 30°C. Heilmann et al. (Citation2012) demonstrated a lower biomass yet higher chitin content in the cell wall of Candida albicans, and the activation of cell wall integrity (CWI) pathway was observed under relatively higher growth temperatures.
Table 2. Variations in the production of glucosamine (GlcN) by A. oryzae NCH-42 at different temperatures.
Tabla 2. Variaciones en la producción de glucosamina (GlcN) por A. oryzae NCH-42 a diferentes temperaturas
3.4. Effect of pH
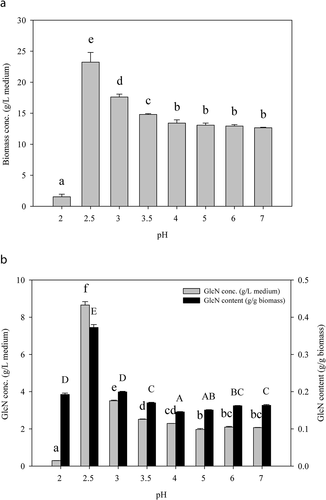
pH is one of the factors that affect the growth and metabolism of fungi. As demonstrated in , the optimal biomass concentration, GlcN concentration, and GlcN content of A. oryzae NCH-42 at pH 2.5 (23.24 g/L medium, 8.66 g/L medium, and 0.37 g/g biomass, respectively) were 1.8, 4.1, and 2.4 times higher compared to that of pH 4 to pH 7. The highest production of GlcN at pH 2.5 was presumably attributed to the strengthening of cell wall under extremely acidic condition. In the condition when the medium was adjusted to pH 2, remarkable (p < .05) reductions in biomass (1.53 g/L medium) and GlcN (0.29 g/L medium) concentrations were noticed. This indicated that this environmental pH is too harsh for this strain. Thus, PDB supplemented with 20 g/L glucose and 30 g/L yeast extract (also known as the modified PDB) with the initial pH adjusted to 2.5 was used as the medium in the following determination.
Figure 3. Variations in (a) biomass concentration and (b) glucosamine (GlcN) production of A. oryzae NCH-42 at different initial pH values. Modified PDB (potato dextrose broth supplemented with 20 g/L glucose and 30 g/L yeast extract) was used as the medium. Bars with different letters are significantly (p < .05) different.
Figura 3. Variaciones en (a) la concentración de biomasa y (b) la producción de glucosamina (GlcN) de A. oryzae NCH-42 a diferentes valores de pH iniciales. Se utilizó como medio el PDB modificado (caldo de dextrosa de papa suplementado con 20 g/L de glucosa y 30 g/L de extracto de levadura). Las barras con letras distintas son significativamente (p < 0.05) diferentes
3.5. Changes in parameters during cultivation
displays the changes in biomass concentration, GlcN concentration, GlcN content, residual sugar concentration, and pH during the cultivation of A. oryzae NCH-42 on the modified PDB medium with pH adjusted to 2.5 at 25°C and 30°C. The biomass concentrations at 25°C and 30°C significantly (p < .05) increased with cultivation time until day 6 (26.97 g/L medium) and day 4 (24.81 g/L medium), respectively ()). Then, the biomass concentrations remained almost constant until the eighth day. In general, GlcN concentration ()) and GlcN content ()) increased rapidly at the first 4 days of cultivation at both 25°C and 30°C and slightly declined afterwards. The optimal GlcN production of A. oryzae NCH-42 with the modified PDB was found to be at the fourth day of cultivation in which the cultivation time was greatly reduced from 7 to 4 days compared to the initial conditions.
Figure 4. Variations in (a) biomass concentration, (b) glucosamine (GlcN) concentration, (c) glucosamine (GlcN) content, (d) residual sugar concentration, and pH during the cultivation of A. oryzae NCH-42 on the modified PDB at 25°C and 30°C. Modified PDB (potato dextrose broth supplemented with 20 g/L glucose and 30 g/L yeast extract) with the initial pH adjusted to pH 2.5 was used as the medium. Bars with different letters are significantly (p< .05) different. Bars with the symbol “*” indicate significant (p < .05) differences between 25°C and 30°C on that particular day of cultivation.
Figura 4. Variaciones en (a) la concentración de biomasa, (b) la concentración de glucosamina (GlcN), (c) el contenido de glucosamina (GlcN), (d) la concentración de azúcar residual y el pH durante el cultivo de A. oryzae NCH-42 en el PDB modificado a 25°C y 30°C. Se utilizó como medio el PDB modificado (caldo de dextrosa de papa suplementado con 20 g/L de glucosa y 30 g/L de extracto de levadura) con el inicial ajustado a pH 2.5. Las barras con letras distintas son significativamente (p < .05) diferentes. Las barras con el símbolo “*” indican diferencias significativas (p < .05) entre 25°C y 30°C en ese día concreto de cultivo
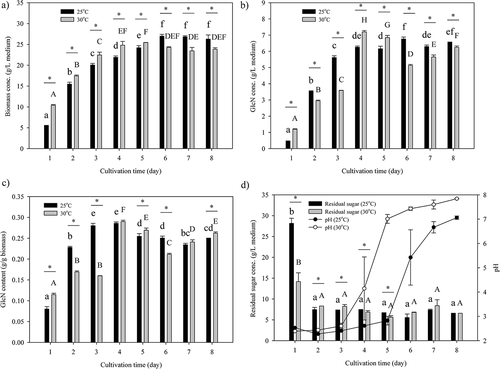
As for the concentrations of residual sugar in the medium ()), both cultivating temperatures decreased sharply on day 2 and were maintained the day after. Meanwhile, the pH of the medium was maintained at approximately 2.5 until day 3. An increasing trend in the pH of the medium was observed at both cultivating temperatures at 25°C and 30°C afterwards. On the eighth day of cultivation, the pH values of the medium reached 7.0 at 25°C and 7.8 at 30°C. Regardless of the cultivating temperature, A. oryzae NCH-42 could modulate the pH to a more neutral environment after 7 days of cultivation.
Aspergillus nidulans, Saccharomyces cerevisiae, C. albicans, and A. niger are some fungal strains that have been identified to possess pH-regulating ability (Peñalva & Arst, Citation2002). The resistance to acidic or high osmotic stress environments of fungi (i.e., Aspergillus species) could bring about morphological changes and structural reinforcement to the cell wall (Klis et al., Citation2002; Peñalva & Arst, Citation2002). This might be due to high levels of transcripts encoding β-1,3-glucanosyltransferases, β-1,3-glucan synthase, and chitin synthases (Yoshimi et al., Citation2016). Peñalva and Arst (Citation2002) pointed out that the acidity-mimicking mutation in pal genes could occur in A. nidulans at acidic environment to modulate the growth environment. Moreover, Sherrington et al. (Citation2017) found that rearrangement of cell wall structure in C. albicans occurred at pH 2 and 4. In order to increase the resistance to acidic stress, the β-glucan and chitin layers within the cell wall of C. albicans were unmasked at pH 4. However, the chitin content of the cell wall elevated significantly, while the mannan fibril layer was reduced at pH 2. To date, nonetheless, such adaptation of fungal cell wall remodelling under acidic stress has not been studied in detail, yet this could be associated with the Rim101/PacC signalling pathway (Hopke et al., Citation2018).
3.6. Determination of GlcN production at modified conditions
exhibits the results of biomass concentration, GlcN concentration, and GlcN content of A. oryzae NCH-42 cultured on either PDB or modified PDB with and without the initial pH adjusted. These three determined parameters were the highest in the modified PDB adjusted to pH 2.5, followed by that at pH 6.5 (without the initial pH adjusted), and the least values were observed solely with PDB. The biomass concentration of A. oryzae NCH-42 was 6.26 g/L medium when cultivated on PDB. By using modified PDB, the biomass concentration increased 2.15 folds without pH adjustment and 3.44 folds with the initial pH adjusted to 2.5. The GlcN content could reach up to 0.31 g/g biomass when the modified PDB adjusted to pH 2.5 was used. This was 7.75 and 2.07 times higher than that of using PDB and modified PDB without pH adjustment.
Table 3. Variations in the production of glucosamine (GlcN) by A. oryzae NCH-42 at different media.
Tabla 3. Variaciones en la producción de glucosamina (GlcN) por A. oryzae NCH-42 en diferentes medios
By adjusting the medium composition and incubating under acidic stress, both biomass concentration and GlcN production of A. oryzae NCH-42 were greatly improved. Several studies have reported GlcN production by fermentation using potent fungal strains with different medium composition and culture conditions. The GlcN content in this study was higher than many reported literatures. Hsieh et al. (Citation2007) reported that a yield of 0.185 g GlcN/g biomass was obtained from Aspergillus sp. BCRC 31742 cultivated at glucose–peptone medium for 7 days. Sitanggang et al. (Citation2010) reported that Asp. sp. BCRC 31742 could produce 0.26 g/g biomass of GlcN when cultured on glucose–peptone medium comprising white granulated sugar, peptone, and methanol for 5 days. Chang et al. (Citation2011) employed the response surface methodology for optimizing the GlcN fermentation medium containing white granulated sugar as well as peptone and reported a higher GlcN yield that could reach up to 0.268 g GlcN/g biomass. Another study on Mucor indicus cultivated on semisynthetic basal medium supplemented with 0.5 g/L potassium dihydrogen phosphate reported that the yield of glucosamine was 0.37 g/L (Mohammadi et al., Citation2013).
3.7. Scanning electron microscopy (SEM) of mycelium
Owing to the elevated GlcN content shown in at pH 2.5, SEM images of A. oryzae NCH-42 were taken to compare the differences at the initial pH unadjusted (pH 6.5) and adjusted to 2.5 (). From the horizontal observations, A. oryzae NCH-42 with the initial pH unadjusted were distorted and collapsed upon sample preparation (i.e., dehydration) ()). On the contrary, the structure of mycelia were straightly elongated, and each hyphae could be observed clearly by adjusting the initial pH ()). As compared to the sample with its initial pH unadjusted, the hyphal surface of A. oryzae NCH-42 were smooth with the acidic stress.
Figure 5. Scanning electron microscopy images of the (a, c) horizontal (magnification 1,200×) and (b, d) cross-sectional (magnification 10,000×) appearance of A. oryzae NCH-42 mycelia cultivated in the modified PDB with the initial pH (a, b) unadjusted and (c, d) adjusted to pH 2.5. Modified PDB (potato dextrose broth supplemented with 20 g/L glucose and 30 g/L yeast extract) was used as the medium and was cultivated for 4 days.
Figura 5. Imágenes de microscopía electrónica de barrido del aspecto (a, c) horizontal (aumento de 1,200×) y (b, d) transversal (aumento de 10,000×) de los micelios de A. oryzae NCH-42 cultivados en el PDB modificado con el pH inicial (a, b) sin ajustar y (c, d) ajustado a pH 2.5. Se utilizó como medio el PDB modificado (caldo de dextrosa de papa suplementado con 20 g/L de glucosa y 30 g/L de extracto de levadura) y se cultivó durante 4 días
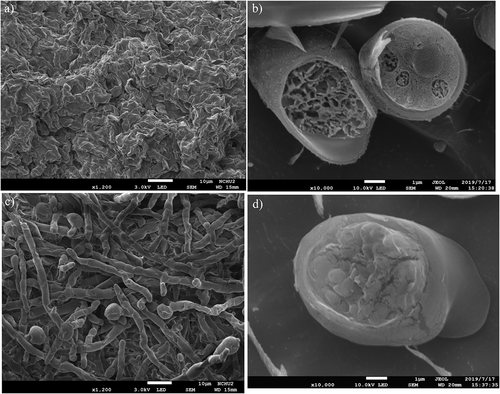
According to ), the cross section of A. oryzae NCH-42 illustrated that the density of internal cross-walls was low and hollow at the initial pH unadjusted, while high and sturdy with the initial pH adjusted to 2.5. The acidic stress treatment led to thicker and solid hyphal walls compared to the neutral environment. As chitin provides networking forces to the cell wall, the upregulation of chitin is a common way for fungi to reinforce their cell wall structure when they are incubated under stresses, like acidic pH environment (Hopke et al., Citation2018).
4. Conclusion
Environmental factors determined in this study, including nitrogen sources, temperature, and pH, appeared to significantly affect cell wall composition of A. oryzae NCH-42. These factors consecutively affected the yield of GlcN produced from A. oryzae NCH-42. Yeast extract was found to be a practical nitrogen source in the enhancement of GlcN yield, while maintaining the growth of A. oryzae at an expected rate. With the favorable ambient temperature at 25–30°C to attain higher productivity of GlcN, environmental pH modulation has been found to be an important factor of the A. oryzae investigated. The optimal biomass concentration and GlcN production of A. oryzae NCH-42 were achieved at pH 2.5. This was accompanied by a morphological change towards stronger and solid structure of the hyphal cell wall as shown by SEM. This study demonstrated that acidic stress significantly enhanced the production of GlcN.
Author contributions
Conceptualization, C.S.C., J.S.L., Y.M.C., and M.C.L.; Methodology, J.S.L., Y.M.C., and M.C.L.; Formal Analysis, Y.M.C., and M.C.L.; Investigation, J.S.L., Y.M.C., M.C.L., and Y.Q.L.; Resources, C.S.C.; Data Curation, Y.M.C., and M.C.L.; Writing – Original Draft Preparation, C.S.C. and Y.M.C.; Writing – Review & Editing, C.S.C., J.S.L., and Y.Q.L.; Visualization, C.S.C., J.S.L., and Y.Q.L.; Supervision, C.S.C.; Project Administration, C.S.C. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We acknowledge the financial support by Grant MOST 105-2221-E-005-075 from the Ministry of Science and Technology, Taiwan, ROC.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Carter, S. B., Nokes, S. E., & Crofcheck, C. L. (2004). The influence of environmental temperature and substrate initial moisture content on Aspergillus niger growth and phytase production in solid-state cultivation. Transactions of the ASAE, 47(3), 945–949. https://doi.org/https://doi.org/10.13031/2013.16073
- Chang, Y., Sitanggang, A. B., & Wu, H. (2011). Optimizing biotechnological production of glucosamine as food ingredient from Aspergillus sp. BCRC 31742. Journal of Food Technology, 9(2), 75–82. https://doi.org/https://doi.org/10.3923/jftech.2011.75.82
- Chhabra, R., Sachdeva, A., Mathur, G., Sharma, P., Goswami, N., Jain, C. K., Sharma, S. K., & Mathur, A. (2014). Enhanced production of fungal chitosan from Aspergillus niger using statistical optimization. Journal of Chitin and Chitosan Science, 2(1), 70–74. https://doi.org/https://doi.org/10.1166/jcc.2014.1066
- Dalirfardouei, R., Karimi, G., & Jamialahmadi, K. (2016). Molecular mechanisms and biomedical applications of glucosamine as a potential multifunctional therapeutic agent. Life Sciences, 152, 21–29. https://doi.org/https://doi.org/10.1016/j.lfs.2016.03.028
- Dhillon, G. S., Kaur, S., Brar, S. K., & Verma, M. (2012). Green synthesis approach: Extraction of chitosan from fungus mycelia. Critical Reviews in Biotechnology, 33(4), 379–403. https://doi.org/https://doi.org/10.3109/07388551.2012.717217
- Di Lonardo, D. P., Van Der Wal, A., Harkes, P., & De Boer, W. (2020). Effect of nitrogen on fungal growth efficiency. Plant Biosystems-An International Journal Dealing with All Aspects of Plant Biology, 154(4), 433–437. https://doi.org/https://doi.org/10.1080/11263504.2020.1779849
- Elsoud, M. M. A., & El Kady, E. M. (2019). Current trends in fungal biosynthesis of chitin and chitosan. Bulletin of the National Research Centre, 43(1), 59. https://doi.org/https://doi.org/10.1186/s42269-019-0105-y
- Ene, I. V., Adya, A. K., Wehmeier, S., Brand, A. C., MacCallum, D. M., Gow, N. A. R., & Brown, A. J. P. (2012). Host carbon sources modulate cell wall architecture, drug resistance and virulence in a fungal pathogen. Cellular Microbiology, 14(9), 1319–1335. https://doi.org/https://doi.org/10.1111/j.1462-5822.2012.01813.x
- Gao, L., Sun, M. H., Liu, X. Z., & Che, Y. S. (2007). Effects of carbon concentration and carbon to nitrogen ratio on the growth and sporulation of several biocontrol fungi. Mycological Research, 111(1), 87–92. https://doi.org/https://doi.org/10.1016/j.mycres.2006.07.019
- Ghormade, V., Pathan, E. K., & Deshpande, M. V. (2017). Can fungi compete with marine sources for chitosan production? International Journal of Biological Macromolecules, 104(Part B), 1415–1421. https://doi.org/https://doi.org/10.1016/j.ijbiomac.2017.01.112
- Hamad, H. O., Alma, M. H., Ismael, H. M., & Goceri, A. (2014). The effect of some sugars on the growth of Aspergillus niger. KSU Journal of Natural Sciences, 17(4), 7–11. https://doi.org/https://doi.org/10.18016/ksujns.28479
- Heilmann, C. J., Sorgo, A. G., Mohammadi, S., Sosinska, G. J., de Koster, C. G., Brul, S., de Koning, L. J., & Klis, F. M. (2012). Surface stress induces a conserved cell wall stress response in the pathogenic fungus Candida albicans. Eukaryotic Cell, 12(2), 254–264. https://doi.org/https://doi.org/10.1128/EC.00278-12
- Hopke, A., Brown, A. J. P., Hall, R. A., & Wheeler, R. T. (2018). Dynamic fungal cell wall architecture in stress adaptation and immune evasion. Trends in Microbiology, 26(4), 284–295. https://doi.org/https://doi.org/10.1016/j.tim.2018.01.007
- Hsieh, J. W., Wu, H. S., Wei, Y. H., & Wang, S. S. (2007). Determination and kinetics of producing glucosamine using fungi. Biotechnology Progress, 23(5), 1009–1016. https://doi.org/https://doi.org/10.1021/bp070037o
- Jun, H., Guangye, H., & Daiwen, C. (2013). Insights into enzyme secretion by filamentous fungi: Comparative proteome analysis of Trichoderma reesei grown on different carbon sources. Journal of Proteomics, 89, 191–201. https://doi.org/https://doi.org/10.1016/j.jprot.2013.06.014
- Klis, F. M., Mol, P., Hellingwerf, K., & Brul, S. (2002). Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiology Reviews, 26(3), 239–256. https://doi.org/https://doi.org/10.1111/j.1574-6976.2002.tb00613.x
- Krappmann, S., & Braus, G. H. (2005). Nitrogen metabolism of Aspergillus and its role in pathogenicity. Medical Mycology, 43(Suppl._1), S31–S40. https://doi.org/https://doi.org/10.1080/13693780400024271
- Latgé, J. P. (2010). Tasting the fungal cell wall. Cellular Microbiology, 12(7), 863–872. https://doi.org/https://doi.org/10.1111/j.1462-5822.2010.01474.x
- Lee, Y. H., Woo, J. H., Choi, S. J., Ji, J. D., & Song, G. G. (2010). Effect of glucosamine or chondroitin sulfate on the osteoarthritis progression: A meta-analysis. Rheumatology International, 30(3), 357–363. https://doi.org/https://doi.org/10.1007/s00296-009-0969-5
- Liao, W., Liu, Y., Frear, C., & Chen, S. (2008). Co-production of fumaric acid and chitin from a nitrogen-rich lignocellulosic material-dairy manure-using a pelletized filamentous fungus Rhizopus oryzae ATCC 20344. Bioresource Technology, 99(13), 5859–5866. https://doi.org/https://doi.org/10.1016/j.biortech.2007.10.006
- Liu, L., Liu, Y., Shin, H. D., Chen, R., Li, J., Du, G., & Chen, J. (2013). Microbial production of glucosamine and N-acetylglucosamine: Advances and perspectives. Applied Microbiology and Biotechnology, 97(14), 6149–6158. https://doi.org/https://doi.org/10.1007/s00253-013-4995-6
- McAlindon, T. E., LaValley, M. P., Gulin, J. P., & Felson, D. T. (2000). Glucosamine and chondroitin for treatment of osteoarthritis: A systematic quality assessment and meta-analysis. Jama, 283(11), 1469–1475. https://doi.org/https://doi.org/10.1001/jama.283.11.1469
- Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31(3), 426–428. https://doi.org/https://doi.org/10.1021/ac60147a030
- Mohammadi, M., Zamani, A., & Karimi, K. (2013). Effect of phosphate on glucosamine production by ethanolic fungus Mucor indicus. Applied Biochemistry and Biotechnology, 171(6), 1465–1472. https://doi.org/https://doi.org/10.1007/s12010-013-0440-7
- Nidheesh, T., Kumar, P. G., & Suresh, P. V. (2015). Enzymatic degradation of chitosan and production of D-glucosamine by solid substrate fermentation of exo-β-D-glucosaminidase (exochitosanase) by Penicillium decumbens CFRNT15. International Biodeterioration & Biodegradation, 97, 97–106. https://doi.org/https://doi.org/10.1016/j.ibiod.2014.10.016
- Nwe, N., Furuike, T., & Tamura, H. (2011). Production, properties and applications of fungal cell wall polysaccharides: Chitosan and glucan. In Jayakumar R., Prabaharan M., & Muzzarelli R. (Eds.), Advances in Polymer Science: Chitosan for Biomaterials II (pp. 187–207). https://doi.org/https://doi.org/10.1007/12_2011_124
- Peñalva, M. A., & Arst, H. N. (2002). Regulation of gene expression by ambient pH in filamentous fungi and yeasts. Microbiology and Molecular Biology Reviews, 66(3), 426–446. https://doi.org/https://doi.org/10.1128/MMBR.66.3.426-446.2002
- Peng, J. W., & Wu, H. S. (2020). Kinetic study of glucosamine production using Aspergillus sydowii BCRC 31742 under solid-state fermentation. Molecules, 25(20), 4832. https://doi.org/https://doi.org/10.3390/molecules25204832
- Sakurai, Y., Misawa, S., & Shiota, H. (1985). Growth and respiratory activity of Aspergillus oryzae grown on solid state medium. Agricultural and Biological Chemistry, 49(3), 745–750. https://doi.org/https://doi.org/10.1271/bbb1961.49.745
- Shahidi, F., Arachchi, J. K. V., & Jeon, Y. J. (1999). Food applications of chitin and chitosans. Trends in Food Science & Technology, 10(2), 37–51. https://doi.org/https://doi.org/10.1016/S0924-2244(99)00017-5
- Sherrington, S. L., Sorsby, E., Mahtey, N., Kumwenda, P., Lenardon, M. D., Brown, I., Ballou, E. R., MacCallum, D. M., & Hall, R. A. (2017). Adaptation of Candida albicans to environmental pH induces cell wall remodelling and enhances innate immune recognition. Public Library of Science Pathogens, 13(5), e1006403. https://doi.org/https://doi.org/10.1371/journal.ppat.1006403
- Sitanggang, A. B., Sophia, L., & Wu, H. S. (2012). MiniReview Aspects of glucosamine production using microorganisms. International Food Research Journal, 19(2), 393–404.
- Sitanggang, A. B., Wu, H. S., & Wang, S. S. (2009). Determination of fungal glucosamine using HPLC with 1-naphthyl isothiocyanate derivatization and microwave heating. Biotechnology and Bioprocess Engineering, 14(6), 819–827. https://doi.org/https://doi.org/10.1007/s12257-009-0105-0
- Sitanggang, A. B., Wu, H. S., Wang, S. S., & Ho, Y. C. (2010). Effect of pellet size and stimulating factor on the glucosamine production using Aspergillus sp. BCRC 31742. Bioresource Technology, 101(10), 3595–3601. https://doi.org/https://doi.org/10.1016/j.biortech.2009.12.084
- Sparringa, R. A., & Owens, J. D. (1999). Glucosamine content of tempe mould, Rhizopus oligosporus. International Journal of Food Microbiology, 47(1–2), 153–157. https://doi.org/https://doi.org/10.1016/S0168-1605(99)00020-3
- Te Biesebeke, R., Levin, A., Sagt, C., Bartels, J., Goosen, T., Ram, A., van den Hondel, C., & Punt, P. (2005). Identification of growth phenotype-related genes in Aspergillus oryzae by heterologous macroarray and suppression subtractive hybridization. Molecular Genetics and Genomics, 273(1), 33–42. https://doi.org/https://doi.org/10.1007/s00438-004-1082-9
- Wang, S., Li, P., Su, J., Liang, R., & Wu, X. (2014). Enhanced glucosamine production with Actinomucor elegans based on stimulating factor of methanol. Indian Journal of Microbiology, 54(4), 459–465. https://doi.org/https://doi.org/10.1007/s12088-014-0485-5
- Yoshimi, A., Miyazawa, K., & Abe, K. (2016). Cell wall structure and biogenesis in Aspergillus species. Bioscience, Biotechnology, and Biochemistry, 80(9), 1700–1711. https://doi.org/https://doi.org/10.1080/09168451.2016.1177446
- Yoshimi, A., Miyazawa, K., & Abe, K. (2017). Function and biosynthesis of cell wall α-1, 3-glucan in fungi. Journal of Fungi, 3(4), 63–84. https://doi.org/https://doi.org/10.3390/jof3040063
Appendix A
Table A1. The additional nitrogen concentration and nitrogen content of different nitrogen sources.
Tabla A1. Concentración de nitrógeno adicional y contenido de nitrógeno de diferentes fuentes de nitrógeno
Table A2. The carbon-to-nitrogen ratio (C/N ratio) in the media supplemented with different yeast extract concentration.
Tabla A2. Ratio carbono-nitrógeno (ratio C/N) en los medios suplementados con diferentes concentraciones de extracto de levadura
