 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Bitter melon is rich in phenolic compounds and has significant potential for commercial use as a functional food material. This study aims to optimize ultrasound-assisted extraction (UAE) conditions for extracting phenolic compounds from bitter melons using a response surface methodology. A central composite design was used to investigate the effects of three independent variables (ethanol concentration, ultrasonic power, and extraction time) on the responses, extraction yield, total polyphenol content (TPC), and antioxidant activity. The optimal UAE conditions were determined to be 59% and 277 W for 14 min, and the corresponding predicted response values for yield, TPC, and antioxidant activity were 33.42%, 18.73 mg GAE/g, and 66.93%, respectively. UAE showed higher extraction yield, TPC, phenolic content, and antioxidant activity than conventional ethanol extraction. These results suggest that bitter melon phenolic compounds obtained by the UAE could be used as natural antioxidant agent or functional ingredient enhancers in suitable food products.
RESUMEN
El melón amargo es rico en compuestos fenólicos y posee un importante potencial para ser usado comercialmente como material alimentario funcional. Con el objetivo de obtener compuestos fenólicos del melón amargo utilizando la metodología de superficie de respuesta, el presente estudio se propuso optimizar las condiciones de extracción asistida por ultrasonidos (UAE). Para ello se utilizó un diseño central compuesto a fin de investigar los efectos de tres variables independientes (concentración de etanol, potencia ultrasónica y tiempo de extracción) en las respuestas, rendimiento de extracción, contenido total de polifenoles (TPC) y actividad antioxidante. Se determinó que las condiciones óptimas de UAE son 59% y 277 W durante 14 minutos, mientras que los correspondientes valores de respuesta previstos para el rendimiento, el TCP y la actividad antioxidante son 33.42%, 18.73 mg GAE/g y 66.93%, respectivamente. Asimismo, se comprobó que la UAE posee mayor rendimiento de extracción, TPC, contenido fenólico y actividad antioxidante que la extracción convencional con etanol. Estos resultados dan cuenta de que los compuestos fenólicos del melón amargo obtenidos mediante UAE pueden utilizarse como agentes antioxidantes naturales o para potenciar ingredientes funcionales en productos alimentarios adecuados.
1. Introduction
The presence of bioactive compounds in the human body enables it to fight against various lifestyle-related disorders, such as cancer incontinence, diabetes mellitus, abdominal pain, kidney (stone), fever, and scabies (Grover & Yadav, Citation2004; Saeed et al., Citation2018). In particular, the ability of phenolic compounds to inhibit oxidation is the characteristics that prevent oxidative damage by various free radicals and reactive oxygen species. Recently, many studies have been conducted to identify antioxidant-rich dietary sources and separate bioactive compounds from natural sources (Gunathilake et al., Citation2019).
Bitter melon (Momordica charantia), a plant belonging to the family Cucurbitaceae, is cultivated worldwide as vegetable and medicine. Bitter melon contains a variety of functional ingredients including flavonoids, polyphenols, glycosides, saponins, alkaloids, triterpenes, and steroids (Saeed et al., Citation2018). Owing to these functional components, bitter melons possess a wide range of pharmacological properties, including antioxidants, antifungals, antidiabetics, and anticancer (Grover & Yadav, Citation2004). The content of polyphenols and other functional substances in bitter melon varies depending on the variety, cultivation area, and harvest period (Horax et al., Citation2010). For example, Horax et al. (Citation2010) reported that bitter melon of immature stage contained higher polyphenols than mature and ripe bitter melons, and seeds showed higher polyphenols than pericarp. The pulp of the ripe fruit and the whole unripe fruit showed the higher amounts of total phenolic compound compared to ripe peel and seed, and whole unripe fruit contained the most type of phenolic compounds (Lopes et al., Citation2020). The phenolic compounds contained in bitter melon showed the highest extraction efficiency when extracted using aqueous ethanol as a solvent (Lopes et al., Citation2018). The ethanol extract of wild-grown bitter melon showed high antioxidant activity and contained three phenolic acids and 11 flavonol glycoside derivatives, among which quercetin-3-O-pentosylhexoside was abundant (Svobodova et al., Citation2017). In addition, because the cell walls of bitter melons are hard and strong, there are significant differences in physiological activity according to the processing and extraction conditions (Park et al., Citation2018). Therefore, it is necessary to determine the optimal conditions for the extraction of phenolic compounds from bitter melon for commercial use as nutraceutical products and natural antioxidants in the food industry.
The process of extracting bioactive compounds from plants for commercial use as functional ingredients is essential in the food industry. Conventional extraction methods, such as heating, boiling, and reflux have been used mainly to extract bioactive compounds from natural materials. However, these extraction methods have problems such as low yield, long extraction time, high-energy consumption, the destruction of useful components through heat, and the use of large amount of extraction solvent (Chemat et al., Citation2012). Recently, some extraction methods, such as ultrasonic extraction, microwave extraction, and supercritical extraction have been employed to improve the extraction process (Nipornram et al., Citation2018; Pandey et al., Citation2018). Ultrasound-assisted extraction (UAE) is used to improve the extraction efficiency of phenolic compounds from various vegetable materials (Ghafoor et al., Citation2009). With this technology, ultrasound waves generate cavitation bubbles in the extracted solvent. When these bubbles collapse, they create local high-temperature and high-pressure conditions which destroy plant cell walls (Rostagno et al., Citation2003). Broken plant cell walls allow the solvent to penetrate the plant tissues, which increase the release of organic compounds from inside plant cells (Sharmila et al., Citation2016).
The UAE method simplifies the extraction process, decreases the extraction time, and requires less solvent compared with conventional extraction methods, thereby enabling the efficient extraction of bioactive compounds (Vilkhu et al., Citation2008). Ultrasonic systems are available in both bath and probe types depending on the location of ultrasound. The ultrasonic probe is advantageous compared to an ultrasound bath because it increases the extraction efficiency of the target component by producing more energy and accelerating the chemical reaction (Wen et al., Citation2018). Many studies have been reported using UAE to extract bioactive compounds from bitter melon (Ahamad et al., Citation2015; Hani et al., Citation2017; Sutanto et al., Citation2015). However, most of these studies use an ultrasonic bath and are not congruous to a study which prepares extracts using ultrasound probes.
The present study aims to optimize the extraction conditions of using an ultrasound probe to effectively recover phenolic compounds with high antioxidant activity from bitter melon for commercial use in the food industry. In addition, the efficiency of the UAE under optimized conditions was compared with that of the ethanol extraction (EE) method, which is a conventional extraction method.
2. Materials and methods
2.1. Material
Bitter melons, grown in Gyeongsan (Korea), were purchased in mid-July (summer). Bitter melons (mature stage, 3–4 weeks post flowering) were selected visually based on the color (green with no yellow color) and overall condition of the fruit (no bruise). The collected bitter melon was washed, seed removed, and the pericarp was used as experimental material. The pericarp was cut into 1 cm thick slices with a knife and freeze-dried. Thereafter, it was ground into a fine powder (about 0.2 mm) using a sample grinder (FM-681 C, Hanil, Incheon, Korea) and stored in a − 40°C freezer (MDF, Sanyo, Tokyo, Japan). Gallic acid, Folin–Ciocalteu phenol reagent and 1,1-diphenyl-2-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Ethanol and all other chemicals were of analytical grade.
2.2. Experimental design
Optimization of the UAE conditions to obtain a phenolic-rich extract with antioxidant activity from bitter melon was performed using a three-factor inscribed central composite design (CCD) with RSM. The design consisted of 20 experimental runs with three levels (−1, 0, 1) for each of the three independent variables: ethanol concentration (X1), ultrasonic power (X2), and extraction time (X3) (). The independent variables were evaluated in the following ranges: ethanol concentration, 30–90%; ultrasonic power, 150–300 W; extraction time, 5–30 min. These ranges were determined based on a preliminary experiment. The extraction temperature and solid–liquid ratio were set to 40°C ± 2 and 1:20 (w/v), respectively, based on the results of the preliminary experiments conducted with reference to the previous report (Pandey et al., Citation2018). Extraction yield, total polyphenol content (TPC), and antioxidant activity were chosen as the responses.
Table 1. Yield, total polyphenol content (TPC) and antioxidant activity of extract obtained from bitter melons by extraction conditions based on central composite design.
Tabla 1. Rendimiento, contenido total de polifenoles (TPC) y actividad antioxidante del extracto de polifenoles del melón amargo según las condiciones de extracción basadas en el diseño central compuesto
2.3. Data analysis
The experimental data were analyzed using the response surface regression procedure of the Statistical Analysis System (version 9.4, SAS Institute, Cary, NC, USA). The responses obtained from the experimental design were fitted to a second-order polynomial model, and regression coefficients were obtained. The second-order polynomial model is given by the following equation:
where is the response variable,
and
are independent variables,
is the intercept coefficient,
is the linear regression coefficient,
is the quadratic coefficient, and
is the interaction coefficient.
The adequacy of the model was predicted using regression analysis (R2) and the analysis of variance (ANOVA) test. The interaction between the independent and dependent surfaces was demonstrated using response surface plots. The predicted extraction conditions were validated under the best extraction conditions obtained using RSM. When the results of the ridge analysis of the SAS regression analysis showed a saddle point, the optimal response was estimated through ridge analysis.
2.4. Ultrasound-assisted extraction (UAE)
The UAE was performed using an ultrasonic probe device (KFS-600 N, Korprotech, Seoul, Korea) equipped with a digital control system to control frequency, sonication time, and temperature. The extraction temperature and solid–liquid ratio were determined based on the results of preliminary experiments performed with reference to a previous report (Pandey et al., Citation2018). Four grams of powdered samples were mixed with 80 mL of aqueous ethanol (1:20 solid/liquid ratio) of varying concentrations, as shown in . Extraction was carried out at a constant frequency of 20 kHz, and temperature of 40 ± 2°C. To prevent the excessive temperature increase during the extraction process, the extracting container was placed in a large beaker filled with ice, and the temperature was monitored using a thermometer. The extract was centrifuged at 12000 × g at −4°C for 20 min, and the collected supernatant was concentrated on a rotary evaporator (N-1000, EYELA, Tokyo, Japan) at 35°C and freeze-dried.
2.5. Ethanol extraction (EE)
To confirm the efficiency of phenolic-rich extract obtained under optimized UAE conditions, it was compared with extract obtained using EE, a conventional extraction method. The EE was carried out according to a previously described study (Kim et al., Citation2009; Oh & Yoon, Citation2017). A dried ground sample (5 g) was mixed with 100 mL of 60% ethanol in an Erlenmeyer flask. The mixture was placed in a shaking water bath (BS-11, JeioTech, Seoul, Korea) at 60°C for 3 h. After extraction, the suspension was filtered and centrifuged at 16270 × g for 20 min. The supernatant was concentrated on a rotary evaporator (R-124, Buchi, Flawil, Switzerland) and freeze-dried.
2.6. Yield determination
The yield of each extract obtained from the UAE was calculated by comparing the weight of the original dried ground bitter melon to that of the freeze-dried bitter melon extract, using the following equation:
2.7. Measurement of total polyphenol content (TPC)
The TPC of the extract was measured using the Folin–Ciocalteu method (Folin & Ciocalteu, Citation1927) with gallic acid as the standard phenol. Briefly, 0.1 mL of sample solution was mixed with 0.1 mL of Folin–Ciocalteu’s phenol reagent and allowed to sit for 3 min. Then, 2 mL of distilled water and 0.2 mL of 10% sodium bicarbonate were added. After incubation at room temperature for 1 h, the absorbance was measured at 725 nm.
2.8. Antioxidant activity by DPPH assay
The ability of the extract to scavenge 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals was determined as described by Jang et al. (Citation2016). The sample solution was diluted to a constant concentration (100 μL) and DPPH solution (200 μL) were added to each well of a 96-well plate. The plate was incubated at 37°C for 30 min, and the absorbance was measured at 517 nm. The DPPH radical scavenging activity was calculated using the following equation:
where Asample and Acontrol are the absorbance of the sample and control, respectively.
2.9. High-performance liquid chromatography (HPLC) analysis of phenolic compounds
The analysis of phenolic compounds in the extracts was conducted using HPLC (Waters 2695; Waters Co., Milford, MA, USA) equipped with a UV/Vis detector (Waters 2489; Waters Co.) and Atlantis dC18 column (4.6 × 150 mm, 5 µm; Waters Co.). Analytical conditions were performed based on the method described by Mradu et al. (Citation2012) with some modification. The sample solution was passed through membrane filters (0.45-µm pore size, Millipore, Billerica, MA, USA) and injected into the HPLC device, and analyzed at 280 nm. The column temperature was maintained at 30°C, and the mobile phase consisted of 1% phosphoric acid (solvent A) and 100% acetonitrile (solvent B) at a flow rate of 1 mL/min. The phenolic compounds in the extract were identified using the retention time of standard compounds. The calibration curves were obtained by the external standard method at four concentrations (0.01–0.5 mg/mL) of each of the following standards: gallic acid, tannic acid, chlorogenic acid, epicatechin, catechin, caffeic acid, and ρ-Coumaric acid. The limit of detection was determined by a signal-to-noise ratio of 3:1, and the limit of quantification was determined by a signal-to-noise ratio of 10:1.
2.10. Statistical analysis for validation
The experimental results for validation and confirming extraction efficiency of the UAE were carried out in triplicate, and the experimental results were expressed as the meanSD. Significant differences between the UAE and EE samples at the significance level p < .05 were analyzed by t-test using SPSS (ver. 21, Chicago, IL, USA).
3. Result and discussion
3.1. Fitting the models
It is necessary to confirm the influence of extraction parameters that may affect the extraction of biologically active compounds from plants. A three-factor, three-level CCD was used to study the effect of various independent variables on the extraction yield, TPC, and antioxidant activity. The experimental design and corresponding response data of yield, TPC, and antioxidant activity under various experimental conditions are presented in . The results of ANOVA for the second-order polynomial models are summarized in . The fitness of the model was studied through a lack of fit test, indicating the suitability of accurately predicting the variability of the model. The lack of fit of the response surface prediction model is problematic when the p-value is less than 0.05, and the model is considered suitable when the p-value is greater than 0.05 (Kong et al., Citation2010). The lack of fit tests showed no influence (p > .05), and suggested that the model has the high accuracy when the yield, TPC, and antioxidant activity for any combination of independent variables are within the range of this study. The linear effect of ethanol concentration (X1) was the only parameter that significantly affected all the responses. The quadratic effects significantly affected the TPC and antioxidant activity. The interaction effects were not significant for any response. Second-order polynomial equations were developed to describe the variations in the responses as functions of the variables. The low p-values (p < .001) of the developed model indicated that the model was statistically significant at the 99% confidence level. The coefficient of determination (R2) was also revealed the quality of the developed model. The R2 values of extraction yield, TPC, and antioxidant activity were 0.93, 0.92, and 0.93, respectively, indicating a good correlation between the predicted and experimental values. Therefore, the developed model, including response surface curves and predictions for optimal extraction, is sufficient for maximizing yield, TPC, and antioxidant activity.
Table 2. Regression coefficient, coefficient of determination (R2) and F-test values of the predicted second-order polynomial models for the yield, total polyphenol content (TPC), and antioxidant activity of bitter melons.
Tabla 2. Coeficiente de regresión, coeficiente de determinación (R2) y valores de la prueba F de los modelos polinomiales de segundo orden previstos para el rendimiento, el contenido total de polifenoles (TPC) y la actividad antioxidante del melón amargo
3.2. Effect of process variables on extraction yield
The extraction yield of bitter melon extracts obtained through the UAE were based on CCD data from 21.49% to 38.00% (). The highest yield (38.00%) was observed in the experimental run 3 with 40% ethanol concentration, 240 W ultrasonic power, and 20 min extraction time. The lowest yield was observed in the experimental run 10 with 90% concentration, 240 W ultrasonic power, and 20 min extraction time. The extraction yield of this study was higher than reported by Nipornram et al. (Citation2018), where the extraction yield of polyphenols obtained under various UAE conditions was 21.50%. The bitter melon extract in this study also showed a higher yield than a previous study, which reported that the maximum yield of bitter melon extract obtained at optimum UAE condition was 28.00% (Hani et al., Citation2017). Multiple regression analysis was performed on the experimental data (), and the coefficients of the model were evaluated for significance (p < .001). The ethanol concentration was positively significant (p < .01) with regard to yield, whereas ultrasonic power and extraction time did not significantly affect the extraction yield. Eliminating all the non-significant terms, the polynomial equation for the yield is:
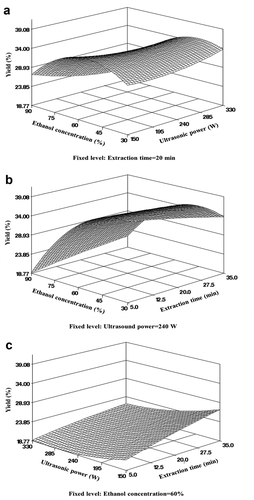
The interactive effects of the independent factors on the yield are shown in . The yield increased as the ethanol concentration increased from 30% to 60% at a constant ultrasonic power; however, the yield decreased as the concentration increased from 60% to 90% ()). Additionally, the extraction yield increased until the ethanol concentration reached 45% and then rapidly decreased with increasing ethanol concentration at a fixed extraction time ()). The ultrasonic power and extraction time did not significantly affect the yield ()). This result is consistent with the study by Gunathilake et al. (Citation2019), which showed an increase in extract yield up to 60% ethanol concentration and the lowest yield at 100% ethanol when extracting polyphenols from Centlla asiatica. The type and concentration of solvents play an important role in the extraction of phenolic compounds and other antioxidant compounds from various natural products (Gunathilake et al., Citation2019). Since water acts as a plant swelling agent and ethanol disrupts the bond between the solutes and plant matrices, aqueous ethanol is more effective in extracting polyphenols (Prasad et al., Citation2011). Analysis of the surface response revealed that the stationary point for extraction yield was a saddle, and a ridge analysis was performed to determine the critical levels of the design variable to produce the maximum response.
Figure 1. Response surface plots of independent variables of bitter melon extract on yield by UAE. (a) Ethanol concentration and ultrasonic power (time 20 min); (b) ethanol concentration and extraction time (ultrasonic power 240 W); (c) ultrasonic power and extraction time (ethanol extraction 60%).
Figura 1. Gráficos de superficie de respuesta de las variables independientes del extracto de melón amargo sobre el rendimiento por UAE. (a) Concentración de etanol y potencia de ultrasonidos (tiempo 20 minutos); (b) concentración de etanol y tiempo de extracción (potencia de ultrasonidos 240 W); (c) potencia de ultrasonidos y tiempo de extracción (extracción de etanol 60%)
3.3. Effect of process variables on TPC
The TPC of bitter melon extracts obtained by the UAE ranged from 14.39 to 19.58 mg GAE/g (). The highest TPC was observed in the experimental run 7 with 80% ethanol concentration, 300 W ultrasonic power, and 10 min extraction time. The lowest TPC was observed in the experimental run 4 with 40% concentration, 300 W ultrasonic power, and 30 min extraction time. The TPC of bitter melon extracts was higher than that of a previous report (Horax et al., Citation2010) in which the TPC of extracts obtained from mature bitter melon with various ethanol concentrations were 6.4–14.8 mg GAE/g. Linear terms that resulted from the regression equation () indicated that all independent variables had a significant (p < .05) positive effect on the TPC, and all quadratics showed a significant (p < .05) negative effect on the TPC. However, the interactive coefficients among the independent factors were insignificant (p > .05). Thus, ethanol concentration, ultrasonic power, and extraction time contributed significantly to the TPC. Eliminating all the non-significant terms, the polynomial equation for the TPC is:
Based on Equationequation (5)(5)
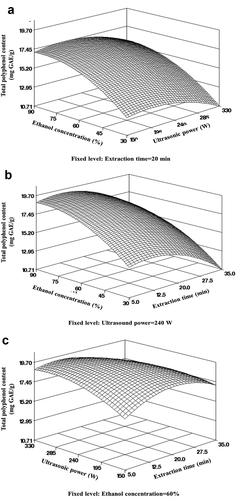
(5) , the three-dimensional surface values were plotted to represent the effects of the UAE variables on the TPC, as shown in . Ethanol concentration (p < .001) and extraction time (p < .01) significantly affected the TPC of bitter melon extracts. The TPC continued to rapidly increase with increasing ethanol concentration when the ultrasonic power increased to nearly 240 W and the extraction time increased to 20 min, but the TPC decreased at ethanol concentrations greater than 60%, ultrasonic powers above 240 W, or after extraction time longer than 20 min ()). The TPC was highest at approximately 20 min of extraction time with a constant ethanol concentration or at a fixed ultrasonic power ()). The TPC differed depending on the ultrasonic power and extraction time, and it was found that the TPC decreased after a certain extraction time. This is attributed to the decomposition and/or oxidation of phenolic compounds by reactive hydroxyl radicals generated from water molecules in the UAE process when treated for a long time under high frequency or ultrasonic power (D’Alessandro et al., Citation2012).
Figure 2. Response surface plots of independent variables of bitter melon extract on total polyphenol content by UAE. (a) Ethanol concentration and ultrasonic power (time 20 min); (b) ethanol concentration and extraction time (ultrasonic power 240 W); (c) ultrasonic power and extraction time (ethanol extraction 60%).
Figura 2. Gráficos de superficie de respuesta de las variables independientes del extracto de melón amargo sobre el contenido total de polifenoles por UAE. (a) Concentración de etanol y potencia ultrasónica (tiempo 20 minutos); (b) concentración de etanol y tiempo de extracción (potencia ultrasónica 240 W); (c) potencia ultrasónica y tiempo de extracción (extracción de etanol 60%)
3.4. Effect of process variables on antioxidant activity
The antioxidant activity of bitter melon extracts obtained by the UAE ranged from 48.42% to 68.30% (). The highest activity was observed in the experimental run 17 with 60% ethanol concentration, 240 W ultrasonic power, and a 20 min extraction time. The lowest activity was observed in the experimental run 4 with 40% concentration, 300 W ultrasonic power, and a 30 min extraction time. In general, TPC has been reported to have a positive correlation with antioxidant, and result of this study showed that the antioxidant activity of bitter melon extracts obtained under various UAE conditions was highly associated with TPC. However, in this study, the extraction conditions with the highest antioxidant activity and the extraction conditions with the highest TPC did not match, most likely due to differences in the types of phenolic compounds in the extracts obtained from each UAE condition. Linear terms that resulted from the regression equation () indicated that ethanol concentration and ultrasonic power had a significant (p < .05) positive effect on antioxidant activity, but extraction time did not have a significant effect on activity. All quadratics among independent factors showed a significant (p < .05) negative effect on antioxidant activity. The polynomial equation showed significant linear and quadratic effects, as follows:
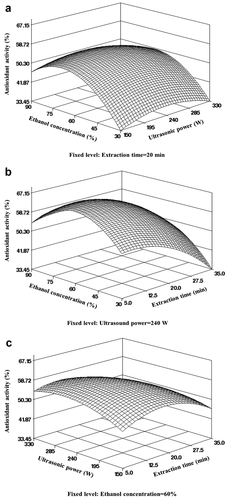
Three-dimensional surface plots of antioxidant activity were similar to those of TPC, and antioxidant activity was significantly (p < .001) affected by ethanol concentration and extraction time. Antioxidant activity rapidly increased with increasing ethanol concentration in the low ultrasonic power range (150–240 W) or at short extraction times (5–15 min); however, the activity increased only up to 60% ethanol concentration when the ultrasonic power was above 240 W or extraction time was longer than 15 min ()). Antioxidant activity also increased with increasing extraction time, and the maximum activity was observed after approximately 15 min at a fixed ultrasonic power ()). It is believed that the extraction time affected the antioxidant activity because increasing the contact time of the solvent with solids improved the diffusion of the compounds. Additionally, the contact time between the solvent and the phenolic compound was increased by extending the extraction time to improve the diffusion of the compound. Thus, the extraction time is believed to influence the antioxidant activity (Ghafoor et al., Citation2009).
Figure 3. Response surface plots of independent variables of bitter melon extract on DPPH antioxidant activity by UAE. (a) Ethanol concentration and ultrasonic power (time 20 min); (b) ethanol concentration and extraction time (ultrasonic power 240 W); (c) ultrasonic power and extraction time (ethanol extraction 60%).
Figura 3. Gráficos de superficie de respuesta de las variables independientes del extracto de melón amargo sobre la actividad antioxidante del DPPH por la UAE. (a) Concentración de etanol y potencia ultrasónica (tiempo 20 minutos); (b) concentración de etanol y tiempo de extracción (potencia ultrasónica 240 W); (c) potencia ultrasónica y tiempo de extracción (extracción de etanol 60%)
3.5. Optimum UAE condition and its validation
Optimal extraction conditions were predicted from the overlapping portions of the superimposed contours of yield, TPC, and antioxidant activity. Based on the analysis of the optimal extraction conditions using Minitab 18 (Minitab Inc., State College, PA, USA), it was predicted that the ideal ethanol concentration was 58–62%, the ideal ultrasonic power was 270–280 W, and the ideal extraction time was 14–16 min. The optimal conditions obtained using Minitab 18 were within the range of those obtained using the contour map. For convenient operation, the optimal extraction parameters were adjusted to 59%, 277 W, and 14 min, and the predicted values of yield, TPC, and antioxidant activity were 33.42%, 18.73 mg GAE/g, and 66.93%, respectively (). The experimental values of yield, TPC, and antioxidant activity were 34.84%, 18.73 mg GAE/g, and 65.32%, respectively, which showed low relative standard deviation (0.53–2.95%). This indicates that the model was well fitted for the extraction of phenolic compounds from bitter melon under optimal UAE conditions, and the designed model was suitable for predicting the optimum extraction conditions.
Table 3. Experimental data of the validation of predicted values at optimal extraction.
Tabla 3. Datos experimentales de la validación de los valores previstos en la extracción óptima
3.6. Comparison of UAE and EE
To confirm the extraction efficiency of the UAE, the phenolic-rich extract obtained by the optimal UAE was compared with the extract obtained by EE, which was representative of conventional extractions. The extraction yield, TPC, phenolic content, and antioxidant activity of the bitter extracts obtained by the UAE and EE are shown in . The bitter extract obtained using UAE showed a significantly higher yield, TPC and DPPH antioxidant activities than that of EE (p < .05). To analyze the phenolic compounds of the UAE and EE, each extract were quantified using HPLC/UV-Vis by comparison to standards. HPLC analysis revealed that UAE contained more types of phenolic compounds and significantly higher phenolic content (p < .05) than EE. For example, in the UAE, gallic acid, tannic acid, chlorogenic acid, catechin, epicatechin, caffeic acid. And ρ-coumaric acid were present at 4.54, 4.14, 1.08, 0.65, 0.61, 0.16 and 0.23 mg/g, respectively. In contrast, EE contained gallic acid (3.05 mg/g), tannic acid (3.82 mg/g), chlorogenic acid (0.69 mg/g), catechin (0.12 mg/g), epicatechin (0.43 mg/g), but not caffeic acid and ρ-coumaric acid. These results suggest that the elution and solubility of intercellular substances increased as the cell walls were destroyed by the bubbles generated by the ultrasonic cavitation. This facilitated the extraction of useful components, including phenolic compounds, from the cells (Horzic et al., Citation2012). These high concentrations of phenolic compounds under optimum UAE conditions are useful for industrial applications, such as nutraceutical product formulations.
Table 4. Yield, total polyphenol content, phenolic content, and antioxidant activity of bitter melon extract by optimization conditions of ultrasound assisted extraction (UAE) and ethanol extraction (EE).
Tabla 4. Rendimiento, contenido total de polifenoles, contenido fenólico y actividad antioxidante del extracto de melón amargo mediante la optimización de las condiciones de extracción asistida por ultrasonidos (UAE) y extracción con etanol (EE)
4. Conclusion
In the present study, the UAE was to extract the phenolic compounds with antioxidant activity from bitter melon. Twenty experiments were performed to investigate three independent variables (ethanol concentration, ultrasonic power, and extraction time) with three levels. RSM was used to analyze the variables for the optimal UAE conditions. Ethanol concentration significantly influenced all dependent variables, and extraction time affected the TPC and antioxidant activity. The most optimal UAE conditions were an ethanol concentration of 59%, ultrasonic power of 277 W, and extraction time of 14 min. The phenolic-rich extracts obtained under optimum conditions provided significantly higher yield, TPC, and antioxidant activity than those obtained by ethanol extraction (EE). In particular, extract by the UAE showed a significantly higher amount of phenolics such as chlorogenic acid, gallic acid, tannic acid, epicatechin, and catechin compared to those obtained by EE. The results indicate that UAE is an efficient method to recover phenolic compounds from bitter melon, and bitter melon extract could be used as a high-value-added new product in the functional food industry.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ahamad, J., Amin, S., & Mir, S. R. (2015). Optimization of ultrasound-assisted extraction of charantin from Momordica charantia fruits using response surface methodology. Journal of Pharmacy & Bioallied Science, 7(4), 304–307. https://doi.org/https://doi.org/10.4103/0975-7406.168032
- Chemat, F., Vian, M. A., & Cravotto, G. (2012). Green extraction of natural products: Concept and principles. International Journal of Molecular Sciences, 13(7), 8615–8627. https://doi.org/https://doi.org/10.3390/ijms13078615
- D’Alessandro, L. G., Kriaa, K., Nikov, I., & Dimitrov, N. K. (2012). Ultrasound assisted extraction of polyphenols from black chokeberry. Separation and Purification Technology, 93(1), 42–47. https://doi.org/https://doi.org/10.1016/j.seppur.2012.03.024
- Folin, O., & Ciocalteu, V. (1927). On tyrosine and tryptophane determinations in proteins. Journal of Biological Chemistry, 73(2), 627–650. https://doi.org/https://doi.org/10.1016/S0021-9258(18)84277-6
- Ghafoor, K., Choi, Y. H., Jeon, Y., & Jo, I. H. (2009). Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from grape (Vitis vinifera) seeds. Journal of Agricultural and Food Chemistry, 57(11), 4988–4994. https://doi.org/https://doi.org/10.1021/jf9001439
- Grover, J. K., & Yadav, S. P. (2004). Pharmacological actions and potential use of Momordica charantia: A review. Journal of Ethnopharmacology, 93(1), 123–132. https://doi.org/https://doi.org/10.1016/j.jep.2004.03.035
- Gunathilake, K. D. P. P., Ranaweera, K. K. D. S., & Rupasinghe, H. P. V. (2019). Response surface optimization for recovery of polyphenols and carotenoids from leaves of Centella asiatica using an ethanol‐based solvent system. Food Science & Nutrition, 7(5), 528–536. https://doi.org/https://doi.org/10.1002/fsn3.832
- Hani, N. M., Torkamani, A. E., Abidin, S. Z., Mahmood, W. A. K., & Juliano, P. (2017). The effects of ultrasound assisted extraction on antioxidative activity of polyphenolics obtained from Momordica charantia fruit using response surface approach. Food Bioscience, 17, 7–16. https://doi.org/https://doi.org/10.1016/j.fbio.2016.11.002
- Horax, R., Hettiarachchy, N., Over, K., Chen, P., & Gbur, E. (2010). Extraction, fractionation and characterization of bitter melon seed proteins. Journal of Agricultural and Food Chemistry, 58(3), 1892–1897. https://doi.org/https://doi.org/10.1021/jf902903s
- Horzic, D., Jambrak, A. R., Belscak-Cvitanovic, A., Komes, D., & Lelas, V. (2012). Comparison of conventional and ultrasound assisted extraction techniques of yellow tea and bioactive composition of obtained extracts. Food and Bioprocess Technology, 5(7), 2858–2870. https://doi.org/https://doi.org/10.1007/s11947-012-0791-z
- Jang, H. R., Liceaga, A. M., & Yoon, K. Y. (2016). Purification, characterisation and stability of an antioxidant peptide derived from sandfish (Arctoscopus japonicus) protein hydrolysates. Journal of Functional Foods, 20, 433–442. https://doi.org/https://doi.org/10.1016/j.jff.2015.11.020
- Kim, S. I., Sim, K. H., Ju, S. Y., & Han, Y. S. (2009). A study of antioxidative and hypoglycemic activities of Omija (Schizandra chinensis Baillon) extract under variable extract conditions. Korean Journal of Food and Nutrition, 22(1), 41–47. http://db.koreascholar.com/article.aspx?code=43163
- Kong, K. W., Ismail, A. R., Tan, S. T., Prasad, K. M. N., & Ismail, A. (2010). Response surface optimisation for the extraction of phenolics and flavonoids from a pink guava puree industrial by-product. International Journal of Food Science & Technology, 45(8), 1739–1745. https://doi.org/https://doi.org/10.1111/j.1365-2621.2010.02335.x
- Lopes, A. P., Galuch, M. B., Petenuci, M. E., Oliveira, J. H., Canesin, E. A., Schneider, V. V. A., & Visentainer, J. V. (2020). Quantification of phenolic compounds in ripe and unripe bitter melons (Momordica charantia) and evaluation of the distribution of phenolic compounds in different parts of the fruit by UPLC–MS/MS. Chemical Paper, 74(8), 2613–2625. https://doi.org/https://doi.org/10.1007/s11696-020-01094-5
- Lopes, A. P., Petenuci, M. E., Galuch, M. B., Schneider, V. V. A., Canesin, E. A., & Visentainer, J. V. (2018). Evaluation of effect of different solvent mixtures on the phenolic compound extraction and antioxidant capacity of bitter melon (Momordica charantia). Chemical Papers, 72(11), 2945–2953. https://doi.org/https://doi.org/10.1007/s11696-018-0461-3
- Mradu, G., Saumyakanti, S., Sohini, M., & Arup, M. (2012). HPLC profiles of standard phenolic compounds present in medicinal plants. International Journal of Pharmacognosy and Phytochemical Research, 4(3), 162–167. http://impactfactor.org/PDF/IJPPR/4/IJPPR,Vol4,Issue3,Article19.pdf
- Nipornram, S., Tochampa, W., & Rattanatraiwong, P. (2018). Optimization of low power ultrasound-assisted extraction of phenolic compounds from mandarin (Citrus reticulata Blanco cv. Sainampueng) peel. Food Chemistry, 241, 338–345. https://doi.org/https://doi.org/10.1016/j.foodchem.2017.08.114
- Oh, M. H., & Yoon, K. Y. (2017). Biological activity of crude polyphenol fractions of Cedrela sinensis isolated using different extraction methods. Korean Journal of Food Science and Technology, 49(4), 438–443. https://doi.org/https://doi.org/10.9721/KJFST.2017.49.4.438
- Pandey, A., Belwal, T., Sekar, K. C., Bhatt, I. D., & Rawal, R. S. (2018). Optimization of ultrasonic-assisted extraction (UAE) of phenolics and antioxidant compounds from rhizomes of Rheum moorcroftianum using response surface methodology (RSM). Industrial Crops and Products, 119, 218–225. https://doi.org/https://doi.org/10.1016/j.indcrop.2018.04.019
- Park, H. S., Moon, B. K., & Kim, S. (2018). Antioxidant and –glucosidase inhibitory activities of fresh bitter melon and change of charantin and lutein content upon brining and blanching treatments of pickling. Korean Journal of Food Science and Technology, 50(4), 430–436. https://doi.org/https://doi.org/10.9721/KJFST.2018.50.4.430
- Prasad, K. N., Hassan, F. A., Yang, B., Kong, K., Ramanan, R. N., Azlan, A., & Ismail, A. (2011). Response surface optimization for the extraction of phenolic compounds and antioxidant capacities of underutilized Mangifera pajang Kosterm peels. Food Chemistry, 128(4), 1121–1127. https://doi.org/https://doi.org/10.1016/j.foodchem.2011.03.105
- Rostagno, A., Palma, M., & Barroso, C. G. (2003). Ultrasound-assisted extraction of soy isoflavones. Journal of Chromatography. A, 1012(2), 119–128. https://doi.org/https://doi.org/10.1016/S0021-9673(03)01184-1
- Saeed, F., Afzaal, M., Niaz, B., Arshad, M. U., & Tufail, T. (2018). Bitter melon (Momordica charantia): A natural healthy vegetable. International Journal of Food Properties, 21(1), 1270–1290. https://doi.org/https://doi.org/10.1080/10942912.2018.1446023
- Sharmila, G., Nikitha, V. S., Ilaiyarasi, S., Dhivya, K., Rajasekar, V., & Kumar, N. M. (2016). Ultrasound assisted extraction of total phenolics from Cassia auriculata leaves and evaluation of its antioxidant activities. Industrial Crops and Products, 84, 13–21. https://doi.org/https://doi.org/10.1016/j.indcrop.2016.01.010
- Sutanto, H., Himawan, E., & Kusumocahyo, S. P. (2015). Ultrasound assisted extraction of bitter gourd fruit (Momordica charantia) and vacuum evaporation to concentrate the extract. Procedia Chemistry, 16, 251–257. https://doi.org/https://doi.org/10.1016/j.proche.2015.12.048
- Svobodova, B., Barros, L., Calhelha, R. C., Heleno, S., Alves, M. J., Walcott, S., Bittova, M., Kuban, V., & Ferreira, I. C. F. R. (2017). Bioactive properties and phenolic profile of Momordica charantia L. medicinal plant growing wild in Trinidad and Tobago. Industrial Crops and Products, 95, 365–373. https://doi.org/https://doi.org/10.1016/j.indcrop.2016.10.046
- Vilkhu, K., Mawson, R., Simons, L., & Bates, D. (2008). Applications and opportunities for ultrasound assisted extraction in the food industry – A review. Innovative Food Science & Emerging Technologies, 9(2), 161–169. https://doi.org/https://doi.org/10.1016/j.ifset.2007.04.014
- Wen, C., Zhang, J., Zhang, H., Dzah, C. S., Zandile, M., Duan, Y., Ma, H., & Luo, X. (2018). Advances in ultrasound assisted extraction of bioactive compounds from cash crops – A review. Ultrasonics-Sonochemistry, 48, 538–549. https://doi.org/https://doi.org/10.1016/j.ultsonch.2018.07.018
