Figures & data
Figure 1. Changes in degrees of hydrolysis (DH) and inhibition activity over hydrolysis time produced from different protease enzymes in different enzyme concentration. A 0.04 E/S, B 0.08 E/S, C 0.1 E/S, and D Trypsin 0.1 E/S degree of hydrolysis with alpha amylase inhibition activity.
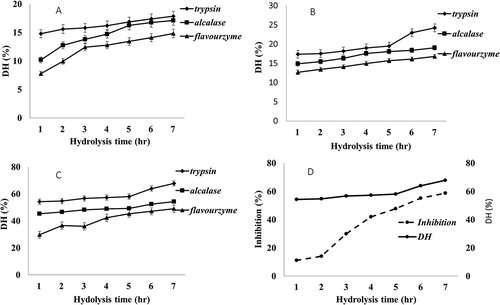
Figure 2. Separation of anti-diabetic peptides from Andrias davidianus protein hydrolysate ultrafiltration fraction by Sephadex G 25 gel filtration load in column 1.6 × 100 cm column. Sample was eluted with water at flow rate of 1 mL/min in the 220 nm wavelength region. Three peptide fractions were obtained according to molecular size distribution, fraction I, II, and III were eluted.
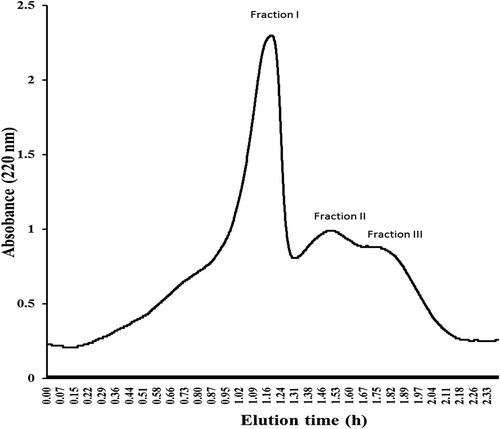
Figure 3. Chromatograms fractions obtain by HPLC with column TSKgel 2000 SWXL 300 mm×7.8mm elute by acetonitrile/water/trichloroacetic acid (TCA), 45/55/0.1 (V/V) show three pattern from fraction I, II and III in with fraction II were corresponding to the target peptide with retaining time 17.557 and 19.656 min.
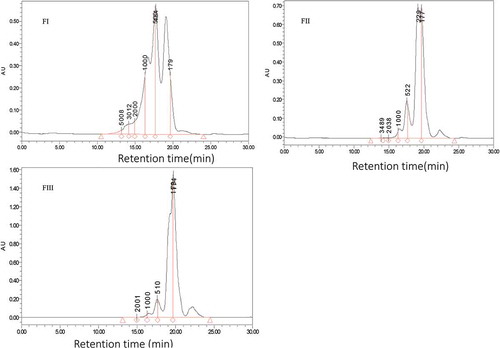
Table 1. Hydrolysis conditions for preparation of protein hydrolysate of Andrias davidianus.
Table 2. Proximate compositions of Andrias davidianus.
Table 3. Biological activities of the peptide fractions separated by hydrolysis, size exclusion chromatography and reverse-phase chromatography. a
Table 4. Amino acid compositions of Andrias davidianus protein hydrolysate.
Table 5. Amino acid sequence and characteristics of purified peptides.