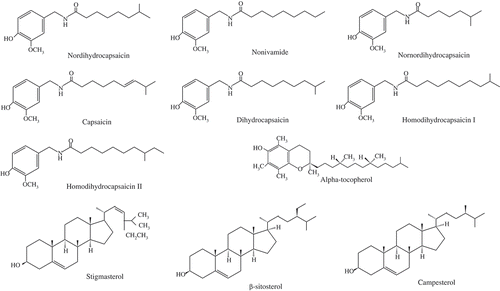
Abstract
A gas chromatography-mass spectrometry method was developed for the quantitative determination of capsaicinoids, vitamin E, and phytosterols in chili peppers using a simple extraction technique for rapid screening. These components were extracted with acetonitrile and were injected into gas chromatography attached with mass spectrometry. The mean recovery values for triplicate analysis were between 90.6–99.7%. Besides major capsaicinoids (capsaicin, dihydrocapsaicin, nordihydrocapsaicin), four more minor capsaicinoids (nonivamide, nornordihydrocapsaicin, homodihydrocapsaicin I and II) were detected in chili samples. α-Tocopherol was also detected in the same run along with three phytosterols (campesterol, γ-sitosterol, and stigmasterol). Variations were observed across the chili sample in all of the constituents except a few minor capsaicinoids. The method was suitable for simultaneous estimation of capsaicinoids, vitamin E, and phytosterols in a single run from any types of pepper. Thus, the method is effective for rapid screening of peppers for its nutraceutical composition using single solvent extraction.
INTRODUCTION
Hot peppers are intensively used as food additives for their pungency, aroma, and colour. The pungent principle of chili fruit is a group of compounds called capsaicinoids, in which the most important components are capsaicin, dihydrocapsaicin, and nordihydrocapsaicin (). Capsaicinoids are vanillylamides of branched chain fatty acids, with 9–11 carbons, of which capsaicin (vanillylamide of 8-methylnontrans-6-enoic acid) and dihydrocapsaicin (vanillylamide of 8 methylnonanoic acid) occur in quantities greater than 80% in chili. These two compounds are the most pungent capsaicinoids, with the equivalent value of 16.1 × 106 Scoville heat unit (SHU).[Citation1] The remaining derivatives are found in very small amounts.[Citation1,Citation2] Capsaicinoids are being produced in glands on the pepper’s placenta and the white ribs present along the sides of a pepper skin. Capsaicinoids are synthesized through the cinnamic acid pathway, and it is thought that their degradation is aided by the action of peroxidase.[Citation3]
In addition to its therapeutic effects as a stimulant and counter irritant, it is being used as a neuropharmacological tool. Capsaicin is known to have great medical value and it has been reviewed to evaluate its effect in treatment of painful conditions, such as rheumatic diseases, cluster headache, painful diabetic neuropathy, postherpetic neuralgia, etc.[Citation4] In recent years, capsaicinoids have been studied and found to be an effective treatment for a number of sensory nerve fiber disorders, including arthritis, cystitis, human immunodeficiency virus, etc.[Citation5,Citation6] They have also been reported as having an antioxidant and antibacterial effect on a certain group of bacteria.[Citation7]
Reverse phase liquid chromatographic method has been used for determination of capsaicin analogues.[Citation8–Citation11] Gas chromatographic (GC) methods were also reported in the literature using packed[Citation12,Citation13] or capillary columns.[Citation14,Citation15] But these methods usually require extensive and long sample cleanup prior to analysis. Most of these methods require derivatization in order to increase the volatility of capsaicinoids, thus increasing the total time of estimation. A gas chromatography-mass spectrometry (GC-MS) method has been reported to analyse these compounds,[Citation16] which include an extra clean-up step for total analysis.
Phytosterols present in vegetables are known to be hypocholesterolemic. These phytochemicals and their derivatives are also potent antioxidants.[Citation17] Phytosterol content in vegetable extract is generally unnoticed. But due to emerging importance of phytosterols in human nutrition, it gained importance. α-Tocopherol content in chili is quite high and it is known to be a significant antioxidant. Thus, the estimation protocol for tocopherol and phytosterols holds a valid reason.[Citation18]
A simple and rapid method was developed including extraction of capsaicinoids as well as sterols and tocopherol with acetonitrile, followed by GC/MS analysis. This procedure allows separation of capsaicinoids, tocopherol, and sterols. Capsaicinoids included three major capsaicinoids: capsaicin, dihydrocapsaicin, and nonivamide, as well as identification of other minor capsaicinoids viz. nordihydrocapsaicin, nornorcapsaicin, homocapsaicin, and homodihydrocapsaicin, reported in the literature.[Citation19] The proposed method does not require derivatization for the estimation and is a compromise between short time analysis and better sensitivity due to inclusion of GC-MS. The present method was validated for determination of capsaicinoids, phytosterols, and tocopherols in chili.
MATERIALS AND METHODS
Reagents and Chemicals
Capsaicin and dihydrocapsaicin were purchased from Sigma Chemical Co. and were of the highest purity. Solvents for GC-MS were chromatographic grade and were obtained from Merck® (India). Water was purified to 18.2 MΩ-cm by means of a bench-top water purifier (Elix, Millipore).
Standards
To prepare stock solution of capsaicin and dihydrocapsaicin, weigh 50 mg of capsaicin, 25 mg dihydrocapsaicin, and put into a 50-mL volumetric flask. Dilute to volume with acetonitrile to give concentrations of 1000 and 500 μg mL−1 capsaicin and dihydrocapsaicin, respectively. Concentrations of working solutions of capsaicin (50–250 ng μL−1) and dihydrocapsaicin (25–250 ng μL−1) were prepared for further analysis.
GC-MS Apparatus and Configuration
GC-MS analysis was carried out using a 7890A GC (Agilent Co., USA) equipped with a HP-5MS column (30 m × 0.25 mm; 0.25 μm; Agilent Co., USA), which was directly connected to a triple axis HED-EM 5975C mass spectrometer (Agilent Co., USA). The injection volume was 1 μl with flow mode in split control. The carrier gas flow was set at 1 ml min−1 helium. Helium (high purity, New Delhi, India) was used as carrier gas at a head pressure of 10 psi. The oven temperature was initially held at 90°C for 1 min. Hereafter, the temperature was raised with a gradient of 5°C min−1 until the temperature reached to 290°C. Other settings were as follows: 250°C interface temperature, 200°C ion source temperature, and electron impact ionization (EI) at 70 eV. Mass spectra were analyzed by both full scan mode and selected ion monitoring (SIM) mode. Raw MS data were processed using the program MSD productivity Chemstation to obtain a purified spectrum by removing residual background contaminants, partially eluting peaks, and column bleed from the spectrum. Structures were confirmed using the library of the instrument. The MS acquisition parameters were: interface temperature, 280°C; ion source temperature, 200°C; electron ionization, 70 eV; full scan mode, 50–550 mass units; solvent delay, 3 min; and E.M voltage, 889.
Sample Preparation
Chili samples were lyophilized before extraction. Samples not subjected to direct analysis were stored at 4°C. One dried chili pepper (1 g) was extracted with 10 mL acetonitrile using a probe ultrasonicator (Sonics®, Vibra cell, ultrasonic processor, 750 Watt, 20 kHz) for 10 min at medium amplitude. An ultrasonicator was run at 50% amplitude and the energy used was 7830 Joules. Repeat the step two more times to get total extraction of capsaicinoids and other phytochemicals. Combine the extracts and centrifuge at 10,000 rpm for 10 min. The extracts were then filtered through a 0.45 μ nylon syringe filter (Millex, India) before chromatographic analysis.
Recovery Study
Recovery study was done by fortifying the chili matrix, known for its content of capsaicin and dihydrocapsaicin with known amounts of capsaicin and dihydrocapsaicin. Fortified samples were extracted following a previous procedure after allowing for completion of capsaicinoid-matrix interaction. Extracted samples were injected in a GC-MS system for quantification. Levels of spiking and recovery values are given in . Results are expressed as percentage of recovery of amounts added for fortification.
TABLE 1 Analytical recoveries of capsaicinoids (mean) added to a chili matrix
RESULTS AND DISCUSSION
Extraction
Acetonitrile was used as extraction solvent because it achieved complete extraction of capsaicinoids along with tocopherol and sterols without extracting much of the interfering materials. The solvent is preferable because the same solvent can be used after centrifugation/filtration for analysis. Another advantage of the extraction method was time saving and it results in extracts suitable for chromatography, especially when using MS detection as it produces clean total ion chromatogram (TIC). Boonkird et al.[Citation20] also used microwave-assisted extraction for analysis of capsaicinoids. Manirakiza et al.[Citation16] used the same solvent for the extraction purpose, whereas Pino et al.[Citation21] used acetonitrile for the same purpose. Kozukue et al.[Citation22] used methanol for extraction of capsaicinoids. Several solvents were evaluated to finalize the solvent for extraction of capsaicinoids.[Citation23] Enzymatic treatments were also used by other investigators to improve the extraction of capsaicinoids from chili fruits.[Citation24]
Many techniques for the extraction of capsaicinoids from peppers have been studied, such as maceration, magnetic stirring, Soxhlet, ultrasound-assisted extraction, extraction by means of supercritical fluids, extraction by pressurized liquids, enzymatic extraction, and microwave-assisted extraction.[Citation8] These techniques are employed in response to the increasing demand for methods that allow the extraction process to be automated, extraction times to be shortened, the consumption of solvents to be reduced, and the exposure of operative personnel to solvents to be minimised. Capsaicinoid and tocopherol was simultaneously estimated using supercritical CO2 and sub-critical pentane extraction followed by HPLC analysis.[Citation25]
GC-MS Characterization
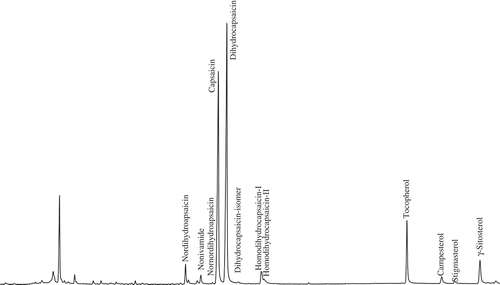
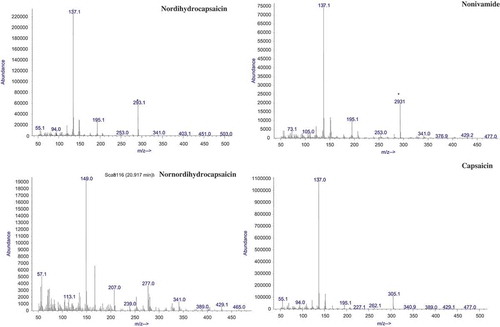
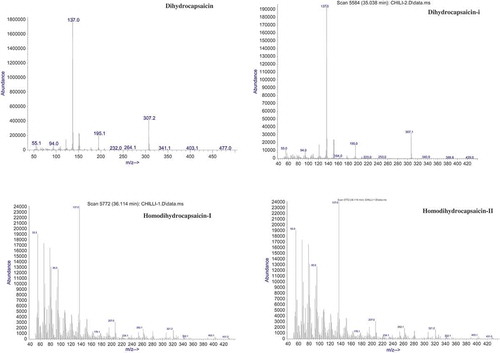
Capsaicinoids were easily separated with the HP-5 MS column, with normal order of elution for a nonpolar stationary phase. showed the representative total ion chromatogram of an extract from a chili sample. Mass spectra of the identified capsaicinoids are given in . No great difference in fragmentation patterns appeared between capsaicinoids except molecular ion peak. Although the most intense ion is m/z = 137, corresponding to the vanillyl moiety, the quantitation was performed using the molecular ions of each compound (m/z = 293, 293, 279, 305, 307, for nordihydrocapsaicin, nonivamide, nornordihydrocapsaicin, capsaicin, and dihydrocapsaicin, respectively).
In a LC-MS study, 3 major and 12 minor capsaicinoids, including nornordihydrocapsaicinoids, were detected in chili pods.[Citation26] Capsaicinoids were identified using GC-MS earlier as such or by derivatization using a different extraction procedure.[Citation16] The present study identified eight capsaicinoids in pepper extracts using gas chromatography/mass selective detection (). GC-MS characterizations of capsaicinoids were reported earlier also.[Citation13,Citation16]
Along with capsaicinoid, three sterols (stigmasterol, campesterol, and β-sitosterol) were also identified and relative content was estimated. Simultaneous gas chromatographic separation of cholestanol, desmosterol, lathosterol, campesterol, and β-sitosterol was achieved using a 60-m length capillary column.[Citation27–Citation30] Sterols were eluted at a temperature above 270°C, which is consistent with the report by Dutta and Normen.[Citation27] Keller and Jahreis[Citation29] reported a GC-MS method for quantitative determination of cholesterol, coprostanol, coprostanone, cholestanol, and bile acids. Another GC-MS method was described by Chevy et al.[Citation30] for determination of cholesterol, lathosterol, 8-dehydrocholesterol, and 7-dehydrocholesterol from amniotic fluid. Tocopherol was also identified and simultaneously analysed in chili extracts by its fragmentation pattern without derivatization. Mass spectra of the identified phytosterols and tocopherols are given in . Tocopherol was estimated earlier after derivatization using the same column.[Citation31] Recently, both GC and HPLC method were used for the estimation of sterols and tocols in vegetable oil.[Citation32]
Recovery Study
Calibration graphs were constructed by plotting the ratio between peak area of analyte and analyte concentration. Equations of plotted curves and correlation coefficients for capsaicin and dihydrocapsaicin are as follows:
TABLE 2 Retention time and mass fragmentation pattern of capsaicinoids, tocopherol, and phytosterols
Quantification
Capsaicinoids, tocopherols, and phytosterols in four chili samples were indicated by the relative content and by comparing the mass spectra between standards and samples. Four major capsaicinoids (capsaicin, dihydropcapsaicin, nonivamide, and nordihydrocapsaicin) were identified and quantified (). Usual variation was observed between chili samples. Two CRCs (capsaicinoid-related compounds) were detected in Chili-I and Chili-II samples. Nornordihydrocapsaicin was identified in Chili-I only. In the other sample, it was found undetectable. Interestingly, DHC-i (isomer of DHC) was also found only in one sample (Chili-II). In terms of α-tocopherol content, Chili-I was found to possess the maximum and the least was found in Chili-II. Campesterol and γ-sitosterol were also detected and variations were detected in chili samples. But, while stigmasterol content was maximum in Chili-I and in the other three samples, it was found not quantifiable (NQ). The present method described in this study was able to estimate major capsaicinoids, tocopherol, and phytosterols in a single run besides identification of minor capsaicinoids. Single solvent extraction, minimum clean up, and quick estimation of major phytochemicals known to be antioxidants made the present investigation novel. Furthermore, no derivatization was required for the estimation of the compounds. The method will be useful for quick screening of chili germplasm for their phytochemical content.
TABLE 3 Quantity and relative content* of capsaicinoids in fresh chili varieties analysed by GC-MS
CONCLUSIONS
The method described in this study was simple, rapid, and allows detection of a range of compounds by using simple extraction followed by direct GC-MS analysis without derivatization. Thus, the simultaneous estimation method of capsaicinoid, tocopherol, and phytosterols made this investigation unique and ideal for quick screening of chili lines used for breeding programme or nutraceutical exploitation. The method ensured high reproducibility. The use of GC-MS for quantitation gives information about the possible interferences, minor constituents, and allows a more accurate quantitation of the capsaicinoid, tocopherol, and sterol content of the chili peppers. The method is suitable for capsaicinoids determination from any type of peppers and is effective for rapid screening of peppers for its nutraceutical composition using single solvent extraction.
ACKNOWLEDGMENTS
The authors are thankful to the Head, Division of Agricultural Chemicals for providing the necessary facilities.
FUNDING
The authors are grateful to the National Agricultural Innovation Project (I.C.A.R.), New Delhi, India for research funding.
REFERENCES
- Krajewska, A.M.; Powers, J.J. Sensory properties of naturally occurring capsaicinoids. Journal of Food Science 1988, 53, 902–905.
- Topuz, A.; Ozdemir, F. Assessment of carotenoids, capsaicinoids and ascorbic acid composition of some selected pepper cultivars (Capsicum annuum L.) grown in Turkey. Journal of Food Composition and Analysis 2007, 20, 596–602.
- Contreras-Padilla, M.C.; Yahia, E.M. Changes in capsaicinoids during development, maturation and senescence of chili peppers and relation with peroxidase activity. Journal of Agricultural and Food Chemistry 1998, 46, 2075–2079.
- Tsuchiya, H. Biphasic membrane effects of capsaicin, an active component in Capsicum species. Journal of Ethnopharmacology 2001, 75, 295–299.
- Robbins, W. Clinical application of capsaicinoids. Clinical Journal of Pain 2000, 16, 86–89.
- Perucka, I.; Materska, M. Phenylalanine ammonia-lyase and antioxidant activities of lipophilic fraction of fresh pepper fruits Capsicum annuum L. Innovative Food Science and Emerging Technologies 2001, 2, 189–192.
- Koffi-Nevry, R.; Kouassi, K.C.; Nanga, Z.Y.; Koussémon, M.; Loukou, G.Y. Antibacterial activity of two bell pepper extracts: Capsicum annuum L. and Capsicum frutescens. International Journal of Food Properties 2012, 15, 961–971.
- Barbero, G.F.; Palma, M.; Barroso, C.G. Determination of capsaicinoids in peppers by microwave-assisted extraction–high-performance liquid chromatography with fluorescence detection. Analytica Chimica Acta 2006, 578, 227–233.
- Cooper, T.H.; Guzinski, J.A.; Fisher, C. Improved HPLC method for determination of major capsaicinoids in Capsicum oleoresins. Journal of Agricultural and Food Chemistry 1991, 39, 2253–2256.
- Kawada, T.; Watanabe, T.; Katsura, K.; Takami, H.; Iwai, K. Formation and metabolism of pungent principle of Capsicum fruits. XV. Microdetermination of capsaicin by liquid chromatography with electrochemical detection. Journal of Chromatography 1985, 329, 99–105.
- Saria, A.; Lembeck, F.; Skofitsch, G. Determination of capsaicin analogues by high performance liquid chromatography. Journal of Chromatography 1981, 208, 41–46.
- Jurenitsch, J.; David, M.; Heresch, F.; Kubelka, W. Detection and identification of new pungent compounds in capsicum fruits. Planta Medica 1979, 36, 61–67.
- Iwai, K.; Suzuki, T.; Fujiwake, H. Simultaneous microdetermination of capsaicin and its four analogues by using high-performance liquid chromatography and gas chromatography-mass spectrometry. Journal of Chromatography 1979, 172, 303–311.
- Gannett, P.; Nagel, D.; Reilly, P.; Lawson, T.; Sharpe, J.; Toth, B. The capsaicinoids: Their separation, synthesis, and metabolism. Journal of Organic Chemistry 1998, 53, 1064–1071.
- Hawer, W.; Ha, J.; Hwang, J.; Nam, Y. Effective separation and quantitative analysis of major heat principles in red pepper by capillary gas chromatography. Food Chemistry 1994, 49, 99–103.
- Manirakiza, P.; Covaci, A.; Schepens, P. Soild phase extraction and gas chromatography with mass spectrometric determination of capsaicin and some analogues from chili peppers. Journal of AOAC International 1999, 82, 1399–1405.
- Wang, R.T.; Wang, T.; Chen, K.; Wang, J.Y.; Zhang, J.P.; Lin, S.R.; Zhu, Y.M.; Zhang, W.M.; Cao, Y.X.; Zhu, C.W.; Yu, H.; Cong, Y.J.; Zheng, S.; Wu, B.Q. Helicobacter pylori infection and gastric cancer: Evidence from retrospective cohort study nested case control study in China. World Journal of Gastroenterology 2002, 8, 1103–1107.
- Ching, L.S.; Mohamed, S. Alpha tocopherol content in 62 edible tropical plants. Journal of Agricultural and Food Chemistry 2001, 49, 3101–3105.
- Thompson, R.Q.; Phinney, K.W.; Welch, M.J.; White, V.E. Quantitative determination of capsaicinoids by liquid chromatography–electrospray mass spectrometry. Analytical and Bioanalytical Chemistry 2005, 381, 1441–1451.
- Boonkird, S.; Phisalaphong, C.; Phisalaphong, M. Ultrasound-assisted extraction of capsaicinoids from Capsicum frutescens on a lab- and pilot-plant scale. Ultrasonic Sonochemistry 2008, 15, 1075–1079.
- Pino, J.; Gonzalez, M.; Ceballos, L.; Centurin-Yah, A.R.; Trujillo-Aguirre, J.; Latowonerie-Moreno, L.; Sauri-Duch, E. Characterization of total capsaicinoids, colour and violate compounds of harbanero chili pepper (Capsicum chinense Jack) cultivars grown in Yucatan. Food Chemistry 2007, 104, 1682–1686.
- Kozukue, N.; Han, J.; Kozukue, E.; Lee, S.; Kim, J.; Lee, K.; Levin, C.E.; Friedman, M. Analysis of eight capsaicinoids in peppers and pepper-containing foods by high-performance liquid chromatography and liquid chromatography-mass spectrometry. Journal of Agricultural and Food Chemistry 2005, 53, 9172–9181.
- Attuquayefio, V.K.; Buckle, K.A. Rapid sample preparation method for HPLC analysis of capsaicinoids in capsicum fruits and oleoresins. Journal of Agricultural and Food Chemistry 1987, 35, 777–779.
- Peusch, M.; Mller Seitz, E.; Petz, M.; Müller, A.; Anklam, E. Extraction of capsaicinoid from chilies (Capsicum frutescens L.) and paprika (Capsicum annuum L.) using supercritical fluids and organic solvents. Zeitschrift Fur Lebensmittel-Untersuchung Und-Forschung A 1997, 204, 351–355.
- Gnayfeed, M.H.; Daood, H.G.; Illes, V.; Biacs, P.A. Supercritical CO2 and subcritical propane extraction of pungent paprika and quantification of carotenoids, tocopherols, and capsaicinoids. Journal of Agricultural and Food Chemistry 2001, 49, 2761–2766.
- Schweiggert, U.; Carle, R.; Schieber, A. Characterization of major and minor capsaicinoids and related compounds in chili pods (Capsicum frutescens L.) by high-performance liquid chromatography/atmospheric pressure chemical ionization mass spectrometry. Analytica Chimica Acta 2006, 557, 236–244.
- Dutta, P.C.; Normen, L. Capillary column gas–liquid chromatographic separation of Δ5-unsaturated and saturated phytosterols. Journal of Chromatography A 1998, 816, 177–184.
- Dyer, R.G.; Heterington, C.S.; Alberti, K.G.M.M.; Laker, M.F. Simultaneous measurement of phytosterols (campesterol and β-sitosterol) and 7-ketocholesterol in human lipoproteins by capillary column gas chromatography. Journal of Chromatography B 1995, 663, 1–17.
- Keller, S.; Jahreis, G. Determination of underivatised sterols and bile acid trimethylsilyl ether methyl esters by gas chromatography–mass spectrometry–single ion monitoring in faeces. Journal of Chromatography B 2004, 13, 199–207.
- Chevy, F.; Humbert, L.; Wolf, C. Sterol profiling of amniotic fluid: A routine method for the detection of distal cholesterol synthesis deficit. Prenatal Diagnosis 2005, 25, 1000–1006.
- Van Pelt, C.K.; Haggarty, P.K.; Brenna, J.T. Quantitative subfemtomole analysis of α-tocopherol and deuterated isotopomers in plasma using tabletop GC/MS/MS. Analytical Chemistry 1998, 70, 4369–4375.
- Hassanien, M.F.R. Plant sterols and tocols profile of vegetable oils consumed in Egypt. International Journal of Food Properties 2013, 16, 574–585.
- Lopez-Hernandez, J.; Oruna Concha, M.J.; Simal Lozano, J.; Gonzalez Castro, M.J.; Vazquez-Blanco, M.E. Determination of capsaicin and dihydrocapsaicin in cayenne pepper and pardon peppers by HPLC. Deutsche Lebensmittel Rundschau 1996, 92, 393–395.